Articles
- Page Path
- HOME > J Mov Disord > Volume 16(3); 2023 > Article
-
Case Report
Rapid-Onset Dystonia and Parkinsonism in a Patient With Gaucher Disease -
Ellen Hertz1*
 , Grisel Lopez1*
, Grisel Lopez1* , Jens Lichtenberg1
, Jens Lichtenberg1 , Dietrich Haubenberger2
, Dietrich Haubenberger2 , Nahid Tayebi1
, Nahid Tayebi1 , Mark Hallett2
, Mark Hallett2 , Ellen Sidransky1
, Ellen Sidransky1

-
Journal of Movement Disorders 2023;16(3):321-324.
DOI: https://doi.org/10.14802/jmd.23074
Published online: June 13, 2023
1Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA
2National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA
- Corresponding author: Ellen Sidransky, MD Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health, Bld 35A Room 1E623, 35A Convent Drive, Bethesda 20892-3708, MD, USA / Tel: +1-301-451-0901 / Fax: +1-301-480-2999 / E-mail: sidranse@mail.nih.gov
- *These authors contributed equally to this work.
Copyright © 2023 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,744 Views
- 96 Download
- 1 Web of Science
ABSTRACT
- Biallelic mutations in GBA1 cause the lysosomal storage disorder Gaucher disease, and carriers of GBA1 variants have an increased risk of Parkinson’s disease (PD). It is still unknown whether GBA1 variants are also associated with other movement disorders. We present the case of a woman with type 1 Gaucher disease who developed acute dystonia and parkinsonism at 35 years of age during a recombinant enzyme infusion treatment. She developed severe dystonia in all extremities and a bilateral pill-rolling tremor that did not respond to levodopa treatment. Despite the abrupt onset of symptoms, neither Sanger nor whole genome sequencing revealed pathogenic variants in ATP1A3 associated with rapid-onset dystonia-parkinsonism (RDP). Further examination showed hyposmia and presynaptic dopaminergic deficits in [18F]-DOPA PET, which are commonly seen in PD but not in RDP. This case extends the spectrum of movement disorders reported in patients with GBA1 mutations, suggesting an intertwined phenotype.
- This 45-year-old right-handed woman presented at age 6 with hepatosplenomegaly and short stature. Splenectomy and bone marrow aspiration were performed, revealing Gaucher cells. She developed extensive skeletal involvement, with spontaneous bone fractures and avascular necrosis of both hips and the left knee. Her genotype was N370S/R463H (p.N409S/R502H). In her late 20s, placenta-derived glucocerebrosidase enzyme replacement therapy became available, and treatment was initiated.
- At 35 years of age, she was switched to recombinant glucocerebrosidase, which was tolerated initially, but during and immediately after her seventh infusion, she described experiencing dizziness, sudden rigidity, and the curling of her fingers and toes. Bilateral upper extremity (UE) tremor developed shortly thereafter. She was unable to ambulate and became wheelchair bound within a month. A year-long hospitalization ensued. She was evaluated by multiple neurologists and psychiatrists without a confirmed diagnosis. Her brain computed tomography (CT) and magnetic resonance imaging (MRI) findings were reported as normal, and fluorodeoxyglucose (FDG)- positron emission tomography (PET) showed no specific abnormalities. Over the years, multiple pharmacological modalities were used to treat her symptoms, which were interpreted as parkinsonism and dystonia of unknown origin. Treatments included two trials of levodopa therapy as well as primidone, biperiden, doxepin, baclofen, mirtazapine, pramipexole, gabapentin, and propranolol. None of these treatments resulted in clinical improvement.
- The patient was evaluated at the National Institutes of Health (NIH) 10 years after the onset of her symptoms. Her past medical history included orthopedic surgical interventions for skeletal manifestations of GD. Additionally, she had a history of panic attacks that responded to antidepressant therapy. At evaluation, her medications included pramipexole, doxepin, domperidone, and imiglucerase. She denied any tobacco, alcohol, or recreational drug use. Her mother, an obligate GBA1 carrier, had a history of PD as well as chronic obstructive pulmonary disease. The patient denied difficulty swallowing but reported mild, diet-controlled constipation. She denied cognitive changes, vivid dreams, or concurrent depression.
- Neurological examination demonstrated perceived intact cognition, fluent normal speech, and normal functioning of cranial nerves II-XII. Her University of Pennsylvania Smell Identification Test (UPSIT) score was 22/40. Masked facies was noted. A bilateral “pill rolling” tremor as well as an action tremor were present, affecting the right UE more than the left. Rigidity was significant in all extremities, with marked dystonia of the neck and shoulder. The range of motion in the lower extremities (LEs) was limited due to her dystonia. Movements of the upper extremities were slow, but objective evidence of bradykinesia in the presence of severe dystonia was unable to be unequivocally confirmed. Muscle stretch reflexes were brisk in the UEs and difficult to assess in the LEs due to contractures. She was unable to stand unassisted and could take only a few steps with the assistance of two individuals. At the NIH, a functional movement disorder expert evaluated the patient and did not find signs supporting a functional etiology. Formal neurocognitive functional testing could not be performed due to a language barrier.
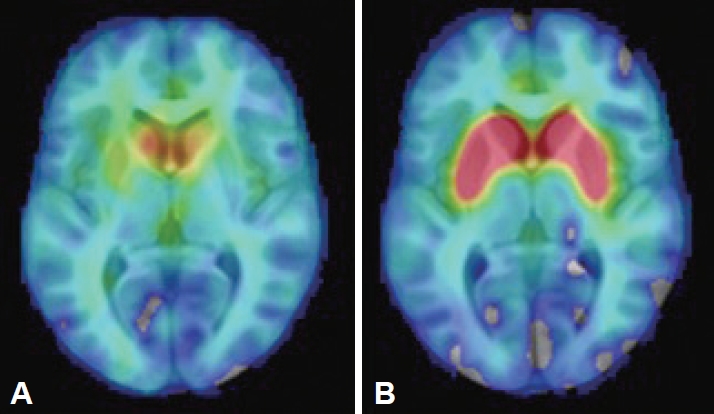
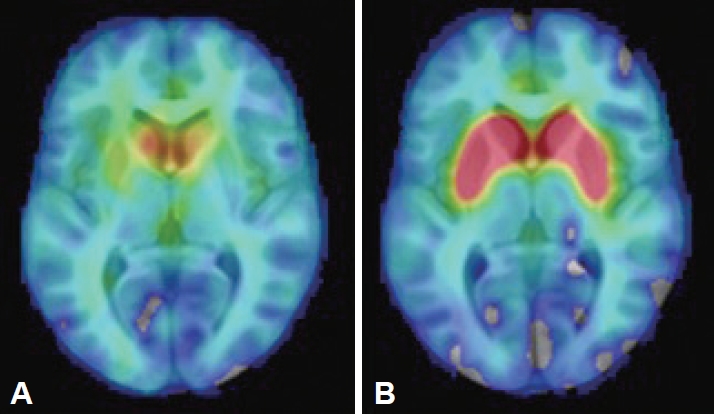
- The findings of brain and abdominal MRI, cerebrospinal fluid analysis, laboratory assessments (including a ceruloplasmin test, a heavy metal screen and serum protein electrophoresis), and electroencephalogram (EEG) were interpreted as normal. Her [18F]-DOPA PET study showed bilateral dopaminergic degeneration (Figure 1). Due to the clinical presentation suggestive of RDP, ATP1A3 was analyzed by both Sanger and whole genome sequencing (WGS), but no pathogenic variants were identified. WGS revealed a pathogenic heterozygote mutation in ARSA (c.542T>G, p.Ile181Ser) when a panel of dystonia-related genes was evaluated. ARSA encodes another lysosomal enzyme, arylsulfatase A, implicated in the recessive disorder metachromatic leukodystrophy, which manifests with the regression of motor skills and spasticity. The findings of one study suggested an association between heterozygous ARSA variants and PD, but the findings were not confirmed in a larger study [14]. No other exonic variants reported as pathogenic in ClinVar were identified in known dystonia-related genes (Supplementary Material in the online-only Data Supplement).
CASE REPORT
- GBA1 mutations have not previously been reported in association with an RDP-like presentation, and this case broadens the spectrum of movement disorders associated with GBA1. The sudden onset of dystonia after a potential trigger, a supporting feature in previous consensus criteria [2], suggested an ATP1A3-related disorder, but the distinct pill-rolling tremor and lack of bulbar symptoms observed in this case have mostly been described in “RDP-like” cases. While this patient had a pathogenic mutation in another lysosomal enzyme, the cause of her rare phenotype has not been proven. Furthermore, dystonia is not described in patients with type 1 GD, although it has rarely been described in patients with neuronopathic (type 3) GD [15].
- This is the first RDP-like case in which presynaptic dopaminergic deficits were observed on PET imaging, a finding frequently associated with PD-like pathology. However, the patients with RDP previously imaged each carried an ATP1A3 mutation (one patient was not genotyped) [16-19]. Patients with GD without PD do not differ from controls in striatal [18F]-fluorodopa uptake [20]. Coexisting dopaminergic degeneration caused by biallelic GBA1 mutations, but clinically masked by severe RDP-like symptoms, could explain the inconsistency, but neuropathological differences between ATP1A3-related and ATP1A3-unrelated cases cannot be excluded. The lack of ATP1A3 mutations in this patient with an atypical RDP-like phenotype may shed light on the cause of the heterogeneity seen in this disorder. We cannot exclude the possibility of a modifying effect of GBA1 mutations resulting in symptoms and findings more commonly associated with PD.
- Last, this case highlights the reliance on clinical judgment in the diagnosis and treatment of movement disorders. The known risk of PD for patients with GD could result in overlooking other movement disorders. Moreover, blended phenotypes can result from the combination of disease-causing variants in different genes. Here, clinical examination resulted in a second diagnosis, with potentially important counseling implications.
DISCUSSION
Supplementary Materials
-
Ethics Statement
The patient was evaluated under a clinical protocol approved by the Institutional Review Board of the National Human Genome Research Institute. The patient provided signed informed consent prior to participation. The authors confirm that they have read the Journal’s position on issues involved in ethical publication and confirm that this work is consistent with those guidelines.
-
Conflicts of Interest
Dietrich Haubenberger is a full-time employee of Neurocrine Biosciences, Inc. (San Diego, CA) and receives royalties from Oxford University Press. Mark Hallett is an inventor of a patent held by National Institutes of Health (NIH) for the H-coil for magnetic stimulation for which he receives license fee payments from the NIH (from Brainsway). He is on the Medical Advisory Boards of Brainsway, QuantalX, and VoxNeuro. The other authors have nothing to disclose.
-
Funding Statement
This work was funded by the Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health.
-
Author Contributions
Conceptualization: Ellen Hertz, Grisel Lopez, Mark Hallett, Ellen Sidransky. Data curation: Ellen Hertz, Grisel Lopez, Nahid Tayebi, Ellen Sidransky. Formal analysis: Ellen Hertz, Grisel Lopez, Jens Lichtenberg. Funding acquisition: Ellen Sidransky. Methodology: Ellen Hertz, Grisel Lopez, Jens Lichtenberg, Nahid Tayebi, Ellen Sidransky. Resources: Ellen Sidransky. Supervision: Jens Lichtenberg, Nahid Tayebi, Ellen Sidransky. Writing—original draft: Ellen Hertz, Grisel Lopez, Jens Lichtenberg. Writing—review & editing: Grisel Lopez, Dietrich Haubenberger, Nahid Tayebi, Mark Hallett, Ellen Sidransky.
Notes
- We thank Dr. Daniel Eisenberg (National Institute of Mental Health) for preparing the positron emission tomography image.
Acknowledgments
- 1. de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, et al. Mutations in the Na+/K+ -ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 2004;43:169–175.ArticlePubMed
- 2. Rosewich H, Sweney MT, DeBrosse S, Ess K, Ozelius L, Andermann E, et al. Research conference summary from the 2014 international task force on ATP1A3-related disorders. Neurol Genet 2017;3:e139. ArticlePubMedPMC
- 3. Haq IU, Snively BM, Sweadner KJ, Suerken CK, Cook JF, Ozelius LJ, et al. Revising rapid-onset dystonia-parkinsonism: broadening indications for ATP1A3 testing. Mov Disord 2019;34:1528–1536.ArticlePubMedPMCPDF
- 4. Brashear A, Dobyns WB, de Carvalho Aguiar P, Borg M, Frijns CJ, Gollamudi S, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain 2007;130:828–835.ArticlePubMed
- 5. Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 2009;361:1651–1661.ArticlePubMedPMC
- 6. Rosenbloom B, Balwani M, Bronstein JM, Kolodny E, Sathe S, Gwosdow AR, et al. The incidence of Parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood Cells Mol Dis 2011;46:95–102.ArticlePubMedPMC
- 7. Bultron G, Kacena K, Pearson D, Boxer M, Yang R, Sathe S, et al. The risk of Parkinson’s disease in type 1 Gaucher disease. J Inherit Metab Dis 2010;33:167–173.ArticlePubMedPMCPDF
- 8. Alcalay RN, Dinur T, Quinn T, Sakanaka K, Levy O, Waters C, et al. Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol 2014;71:752–757.ArticlePubMedPMC
- 9. Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain 2013;136(Pt 2):392–399.ArticlePubMed
- 10. Neumann J, Bras J, Deas E, O’Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 2009;132(Pt 7):1783–1794.ArticlePubMedPMC
- 11. Mitsui J, Matsukawa T, Sasaki H, Yabe I, Matsushima M, Dürr A, et al. Variants associated with Gaucher disease in multiple system atrophy. Ann Clin Transl Neurol 2015;2:417–426.ArticlePubMedPMCPDF
- 12. Potnis KC, Flueckinger LB, DeArmey SM, Alcalay RN, Cooney JW, Kishnani PS. Corticobasal syndrome in a man with Gaucher disease type 1: expansion of the understanding of the neurological spectrum. Mol Genet Metab Rep 2018;17:69–72.ArticlePubMedPMC
- 13. Oliveira LM, Rastin T, Nimmo GAM, Ross JP, Dion PA, Zhang M, et al. Occurrence of amyotrophic lateral sclerosis in Type 1 Gaucher disease. Neurol Genet 2021;7:e600. ArticlePubMedPMC
- 14. Makarious MB, Diez-Fairen M, Krohn L, Blauwendraat C, Bandres-Ciga S, Ding J, et al. ARSA variants in α-synucleinopathies. Brain 2019;142:e70. ArticlePubMedPMCPDF
- 15. Machaczka M, Paucar M, Björkvall CK, Smith NJC, Cox TM, Forsgren L, et al. Novel hyperkinetic dystonia-like manifestation and neurological disease course of Swedish Gaucher patients. Blood Cells Mol Dis 2018;68:86–92.ArticlePubMed
- 16. Svetel M, Ozelius LJ, Buckley A, Lohmann K, Brajković L, Klein C, et al. Rapid-onset dystonia-parkinsonism: case report. J Neurol 2010;257:472–474.ArticlePubMedPDF
- 17. Zanotti-Fregonara P, Vidailhet M, Kas A, Ozelius LJ, Clot F, Hindié E, et al. [123I]-FP-CIT and [99mTc]-HMPAO single photon emission computed tomography in a new sporadic case of rapid-onset dystonia-parkinsonism. J Neurol Sci 2008;273:148–151.ArticlePubMed
- 18. Kamphuis DJ, Koelman H, Lees AJ, Tijssen MA. Sporadic rapid-onset dystonia-parkinsonism presenting as Parkinson’s disease. Mov Disord 2006;21:118–119.ArticlePubMed
- 19. Deutschlander A, Asmus F, Gasser T, Steude U, Bötzel K. Sporadic rapidonset dystonia-parkinsonism syndrome: failure of bilateral pallidal stimulation. Mov Disord 2005;20:254–257.ArticlePubMedPDF
- 20. Lopez G, Eisenberg DP, Gregory MD, Ianni AM, Grogans SE, Masdeu JC, et al. Longitudinal positron emission tomography of dopamine synthesis in subjects with GBA1 mutations. Ann Neurol 2020;87:652–657.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite