Articles
- Page Path
- HOME > J Mov Disord > Volume 16(3); 2023 > Article
-
Letter to the editor
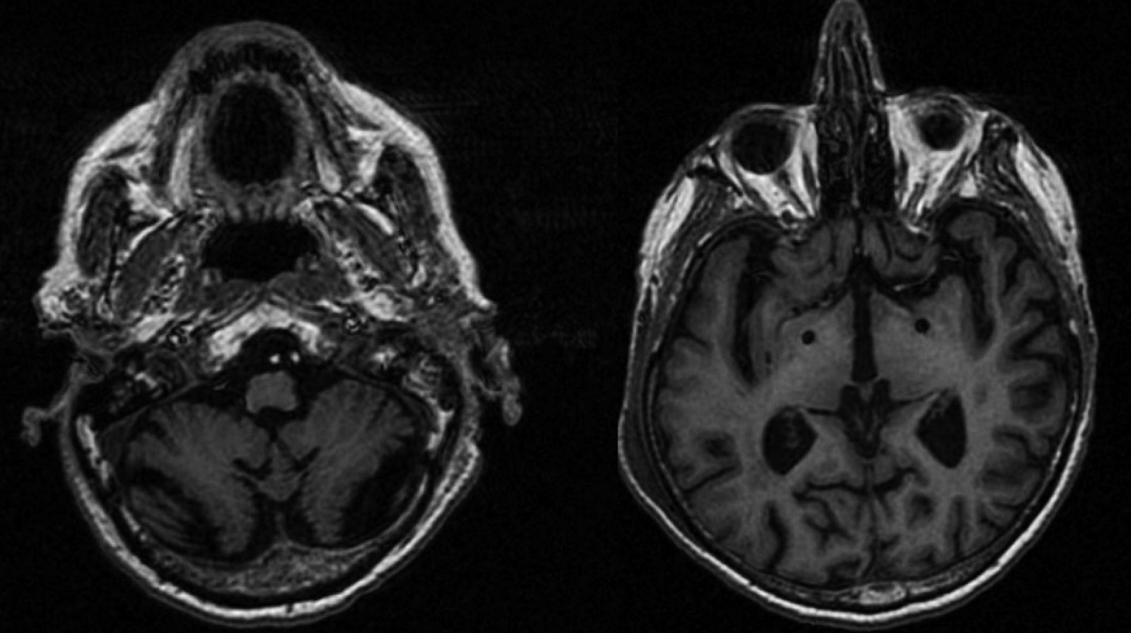
Pallidal Deep Brain Stimulation for Refractory Celiac-Related Myoclonus -
Jinyoung Youn1,2,3,4
 , Elizabeth Slow3,4
, Elizabeth Slow3,4 , Robert Chen3,4,5
, Robert Chen3,4,5 , Andres M. Lozano6,7
, Andres M. Lozano6,7 , Alfonso Fasano3,4,6,8
, Alfonso Fasano3,4,6,8

-
Journal of Movement Disorders 2023;16(3):325-327.
DOI: https://doi.org/10.14802/jmd.23006
Published online: June 9, 2023
1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Neuroscience Center, Samsung Medical Center, Seoul, Korea
3Edmond J. Safra Program in Parkinson’s Disease, Morton and Gloria Shulman Movement Disorders Clinic, Toronto Western Hospital, University Health Network, Toronto, ON, Canada
4Division of Neurology, University of Toronto, Toronto, ON, Canada
5Division of Brain, Imaging and Behavior, Systems Neuroscience, University Health Network, University of Toronto, Toronto, ON, Canada
6Krembil Brain Institute, Toronto, ON, Canada
7Division of Neurosurgery, University of Toronto, Toronto, ON, Canada
8Center for Advancing Neurotechnological Innovation to Application (CRANIA), Toronto, ON, Canada
- Corresponding author: Alfonso Fasano MD, PhD Movement Disorders Center, Toronto Western Hospital, 399 Bathurst St, 7McL410, Toronto M5T 2S8, ON, Canada / Tel: +1-416-603-5800 / Fax: +1-416-603-5004 / E-mail: alfonso.fasano@uhn.ca
Copyright © 2023 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Pallidal deep brain stimulation for patients with myoclonus-dystonia without SGCE mutations
Jun Ikezawa, Fusako Yokochi, Ryoichi Okiyama, Ayako Isoo, Takashi Agari, Tsutomu Kamiyama, Akihiro Yugeta, Maya Tojima, Takashi Kawasaki, Katsushige Watanabe, Satoko Kumada, Kazushi Takahashi
Journal of Neurology.2024;[Epub] CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite