Articles
- Page Path
- HOME > J Mov Disord > Early view > Article
-
Letter to the editor
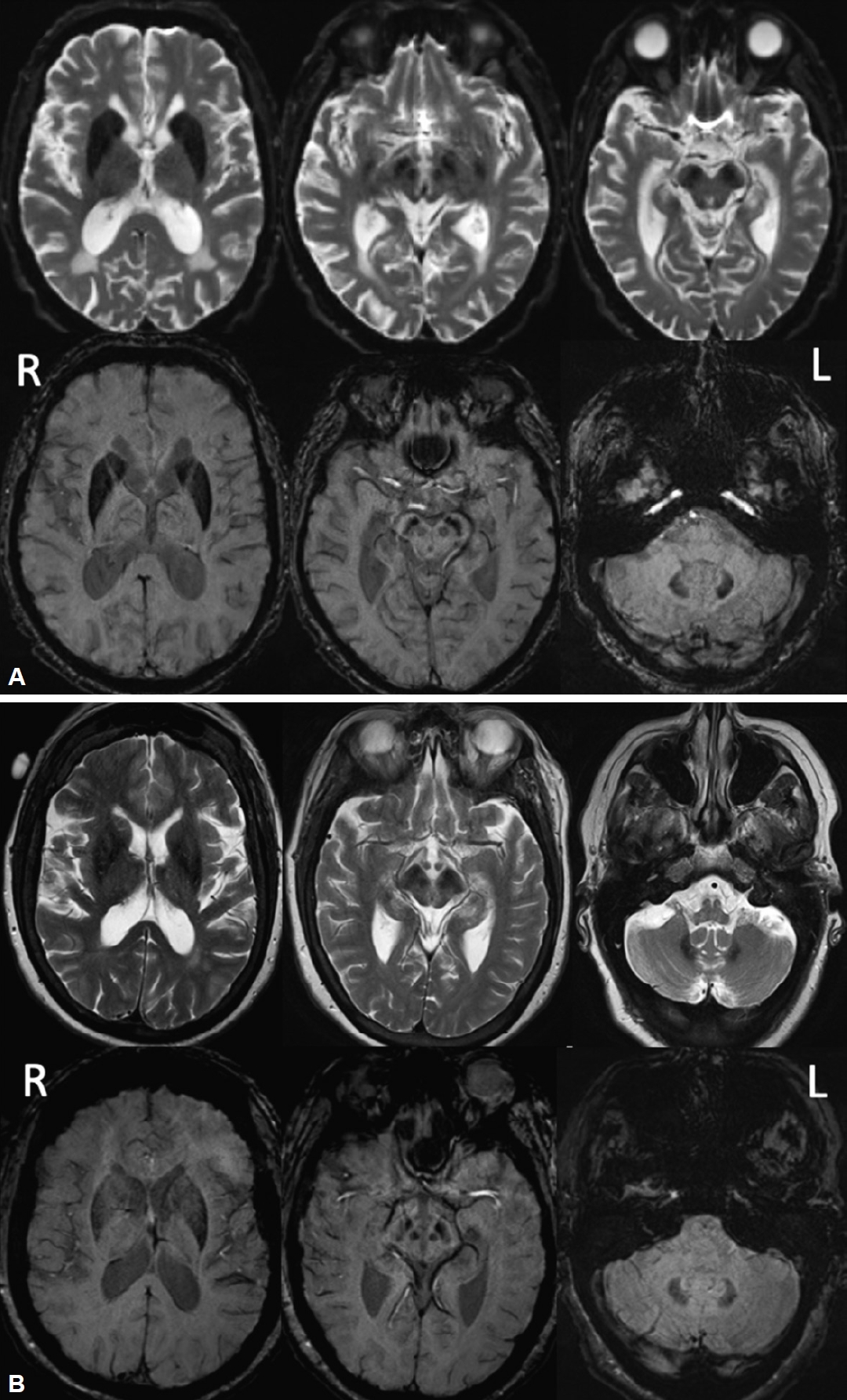
Basal Ganglia Syndrome in a Male With an XK Gene Variant but Without XK Disease (McLeod Syndrome) -
Jeryl Ritzi T. Yu1,2

 , Ruth H. Walker3
, Ruth H. Walker3 , Adrian Danek4
, Adrian Danek4 , Connie M. Westhoff5
, Connie M. Westhoff5 , Sunitha Vege5
, Sunitha Vege5 , Ilia Itin6
, Ilia Itin6
-
>
Epub ahead of print
DOI: https://doi.org/10.14802/jmd.23196
Published online: January 8, 2024
1Institute for Neurosciences, St. Luke’s Medical Center, Quezon City and Global City, Philippines
2University of the East, Ramon Magsaysay Memorial Medical Center, Quezon City, Philippines
3James J. Peters VA Medical Center, Bronx; Mount Sinai School of Medicine, New York, NY, USA
4Department of Neurology, LMU University Hospital, LMU Munich, Munich, Germany
5New York Blood Center, New York, NY, USA
6Center for Neurological Restoration, Cleveland Clinic, Cleveland, OH, USA
- Corresponding author: Jeryl Ritzi T. Yu, MD Institute for Neurosciences, St. Luke’s Medical Center, 279 E. Rodriguez Sr. Ave, Quezon City 1112, Philippines / Tel: +63-9204812608 / E-mail: jrtyu@stlukes.com.ph
Copyright © 2024 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 530 Views
- 40 Download
-
Ethics Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the index patient.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
Western blotting analysis for chorein was performed by G. Kwiatkowski and Dr. Benedikt Bader with the financial support of the Advocacy for Neuroacanthocytosis Patients and of the ERA-net E-Rare consortium EMINA (European Multidisciplinary Initiative on Neuroacanthocytosis; BMBF 01GM1003) in the labs of Profs. Hans Kretzschmar (Neuropathology) and Adrian Danek (Neurology) at Ludwig-Maximilians-Universität Munich, Germany. This study did not receive additional funding.
-
Author contributions
Conceptualization: Jeryl Ritzi T. Yu, Ruth H. Walker, Ilia Itin. Formal analysis: Adrian Danek, Connie M. Westhoff, Sunitha Vege. Project administration: Jeryl Ritzi T. Yu, Ruth H. Walker, Ilia Itin. Writing—original draft: Jeryl Ritzi T. Yu. Writing—review & editing: all authors.
Notes
- 1. Roulis E, Hyland C, Flower R, Gassner C, Jung HH, Frey BM. Molecular basis and clinical overview of McLeod syndrome compared with other neuroacanthocytosis syndromes: a review. JAMA Neurol 2018;75:1554–1562.ArticlePubMed
- 2. Jung HH, Danek A, Walker RH, Frey BM, Peikert K. McLeod neuroacanthocytosis syndrome [Updated 2021 Sep 16]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al. GeneReviews® [Internet]. Seattle: University of Washington c1993-2024. [accessed on 2023 Aug 1]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1354/.
- 3. Walker RH, Danek A, Uttner I, Offner R, Reid M, Lee S. McLeod phenotype without the McLeod syndrome. Transfusion 2007;47:299–305.ArticlePubMed
- 4. Park JS, Hu Y, Hollingsworth NM, Miltenberger-Miltenyi G, Neiman AM. Interaction between VPS13A and the XK scramblase is important for VPS13A function in humans. J Cell Sci 2022;135:jcs260227.ArticlePubMedPMCPDF
- 5. Guillén-Samander A, Wu Y, Pineda SS, García FJ, Eisen JN, Leonzino M, et al. A partnership between the lipid scramblase XK and the lipid transfer protein VPS13A at the plasma membrane. Proc Natl Acad Sci U S A 2022;119:e2205425119.PubMedPMC
- 6. Floch A, Lomas-Francis C, Vege S, Westhoff CM. Three new XK alleles; two associated with a McLeod RBC phenotype. Transfusion 2021;61:E69–E70.PubMed
- 7. Klempír J, Roth J, Zárubová K, Písacka M, Spacková N, Tilley L. The McLeod syndrome without acanthocytes. Parkinsonism Relat Disord 2008;14:364–366.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite

