Articles
- Page Path
- HOME > J Mov Disord > Early view > Article
-
Brief communication
Accessibility of Device-Aided Therapies for Persons With Parkinson’s Disease in Poland -
Katarzyna Smilowska1,2

 , Tomasz Pietrzykowski3
, Tomasz Pietrzykowski3 , K. Ray Chaudhuri4,5
, K. Ray Chaudhuri4,5 , Bastiaan R. Bloem2
, Bastiaan R. Bloem2 , Daniel J. van Wamelen2,5,6
, Daniel J. van Wamelen2,5,6
-
>
Epub ahead of print
DOI: https://doi.org/10.14802/jmd.23172
Published online: November 20, 2023
1Department of Neurology, 5th Regional Hospital, Sosnowiec, Poland
2Radboud University Medical Centre; Donders institute for Brain, Cognition and Behaviour; Department of Neurology; Centre of Expertise for Parkinson & Movement Disorders; Nijmegen, The Netherlands
3Research Centre for Public Policy and Regulatory Governance, Faculty of Law and Administration, University of Silesia in Katowice, Katowice, Poland
4King’s College London, Institute of Psychiatry, Psychology & Neuroscience, Department of Basic and Clinical Neuroscience, London, UK
5Parkinson’s Foundation Center of Excellence, King’s College Hospital, Denmark Hill, London, UK
6King’s College London, Institute of Psychiatry, Psychology & Neuroscience, Department of Neuroimaging, London, UK
- Corresponding author: Katarzyna Smilowska, MD, PhD Department of Neurology, 5th Regional Hospital, Sosnowiec 41-200, Poland / Tel: +48-323682689 / Fax: +48-323682032 / E-mail: Kasia.smilowska@gmail.com
Copyright © 2024 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 586 Views
- 57 Download
ABSTRACT
-
Objective
- Access to care for people with Parkinson’s disease (PD), particularly to device-aided therapies (DAT), is not equally distributed. The objective was to analyze accessibility to DAT (deep brain stimulation, intraduodenal levodopa pump therapy, and apomorphine pump therapy) in Poland.
-
Methods
- We analyzed the distribution of DAT use in Poland by determining the number of persons with PD receiving one of the three DATs during 2015–2021.
-
Results
- In 2021, the number of persons receiving DAT in Poland was 0.56% of the total PD population, increasing from 0.21% in 2015. Overall, deep brain stimulation was the preferred DAT in Poland, but strong regional differences in the use of the other DATs were observed. Accessibility to DAT was negatively associated with average annual income (p < 0.001).
-
Conclusion
- Access to DAT for persons with PD in Poland is still limited, and strong regional differences in accessibility were observed, although its general increase over the last decade is encouraging.
- Parkinson’s disease (PD) is a complex neurodegenerative disease, and persons with PD present with motor and nonmotor symptoms [1]. Although in the early stages of PD, motor symptoms can often be effectively managed with relatively affordable oral pharmacotherapy, in advanced stages, this treatment becomes more complex and costly. As the disease progresses, the therapeutic window for oral dopaminergic treatment narrows, and areas that are nondopaminergic in nature become more involved, with some symptoms responding poorly to dopaminergic pharmacotherapy [2,3]. One possible strategy is to optimize oral pharmacotherapy through dose fractioning, but it is widely believed that a form of therapy with more continuous dopaminergic or nondopamingeric stimulation is a better alternative [4].
- There are currently three types of device-aided therapies (DAT) available, namely, deep brain stimulation (DBS), intraduodenal levodopa pump therapy (LCIG), and apomorphine pump therapy (CSAI). Once the criteria for initiation are met, it is crucial to carefully consider the timing of implementation [5]. This shift from oral medication to continuous dopaminergic stimulation [4] has beneficial effects on both motor and nonmotor symptoms and therefore improves quality of life [6]. Given the latter, as well as the cost-effectiveness of most DATs [6] and the reduction in caregiver burden [7], knowledge about the accessibility of DAT is crucial to provide equal opportunities to all people with PD and maintain quality of life.
- Data on the accessibility of DAT, however, are lacking and are likely to be needed to further optimize their use. Therefore, we aimed to analyze the use of DAT and its accessibility in Poland.
INTRODUCTION
- We obtained the number of persons with PD (International Classification of Diseases 10th Revision [ICD-10] code G20) from the National Health Fund (NHF) database in Poland [8]. People who underwent DBS were identified using code A04 (5.06.00.0001413). In Poland, pump therapies are exclusively available through the NHF’s drug program, which is limited to specific centers. Approval from a central authority is needed for eligible patients, but centers use a separate NHF reporting system, causing delays in the submission of data to the central registry. To address this, we relied on company-provided data, which are considered more reliable and are based on drug requisition records, from Ever Pharma and AbbVie, who provide CSAI and LCIG, respectively, in Poland. None of these companies were involved in data collection or interpretation.
- We extracted data from the NHF database, including the year of reporting, the total number of people with ICD-10 code G20 for each year and per region, sex-specific breakdowns, the population size, and the number of individuals on specific DAT. The number of registered neurologists was obtained from the Polish Chamber of Physicians and Dentists (https://nil.org.pl), but data on movement disorder neurologists at regional levels were not available.
- We calculated the ratio of DAT to neurologists in different regions of Poland to gauge the distribution of this therapy. This indicator was chosen because it has been previously suggested that the number of neurologists could be a factor of influence in relation to awareness and access of persons with Parkinson’s disease for DAT [7]. Additionally, to better understand regional differences in accessibility to DATs, we correlated the number of people with PD on DATs per neurologist considering the percentage of people with PD who completed higher education [9] and average annual income [9].
- Data were summarized descriptively and are presented as the mean ± standard deviation or number (percentage), unless otherwise specified. Group comparisons were performed by using the Kruskal–Wallis test, and correlations were performed through Spearman’s rank sum test with Bonferroni correction for multiple testing. All analyses were performed using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, USA).
MATERIALS & METHODS
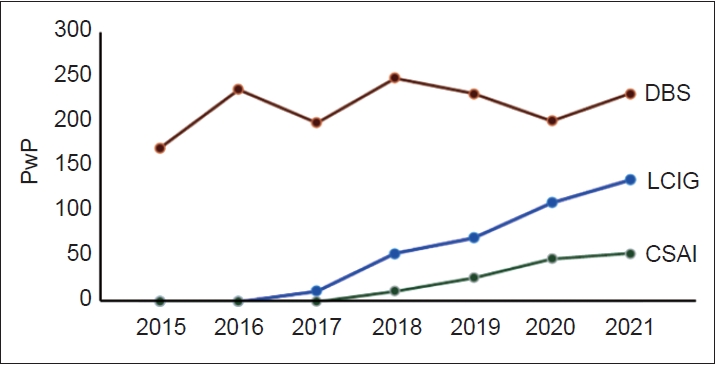
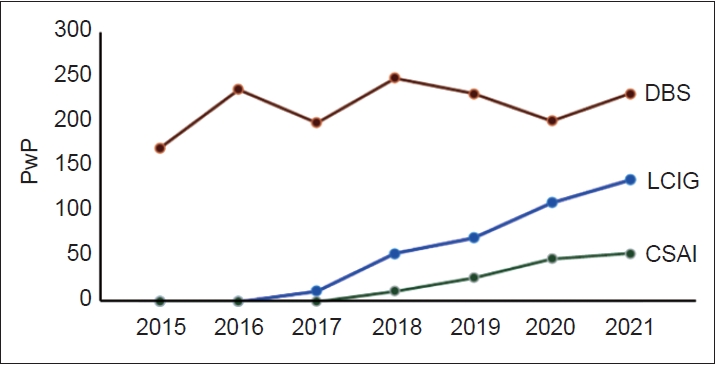
- The use of DAT over the 2015–2021 period is summarized in Figure 1, showing increased availability of these therapies in Poland across this period. In 2021, the number of persons with PD on DAT was 257, which represents 0.56% of the total number of persons with PD in Poland.
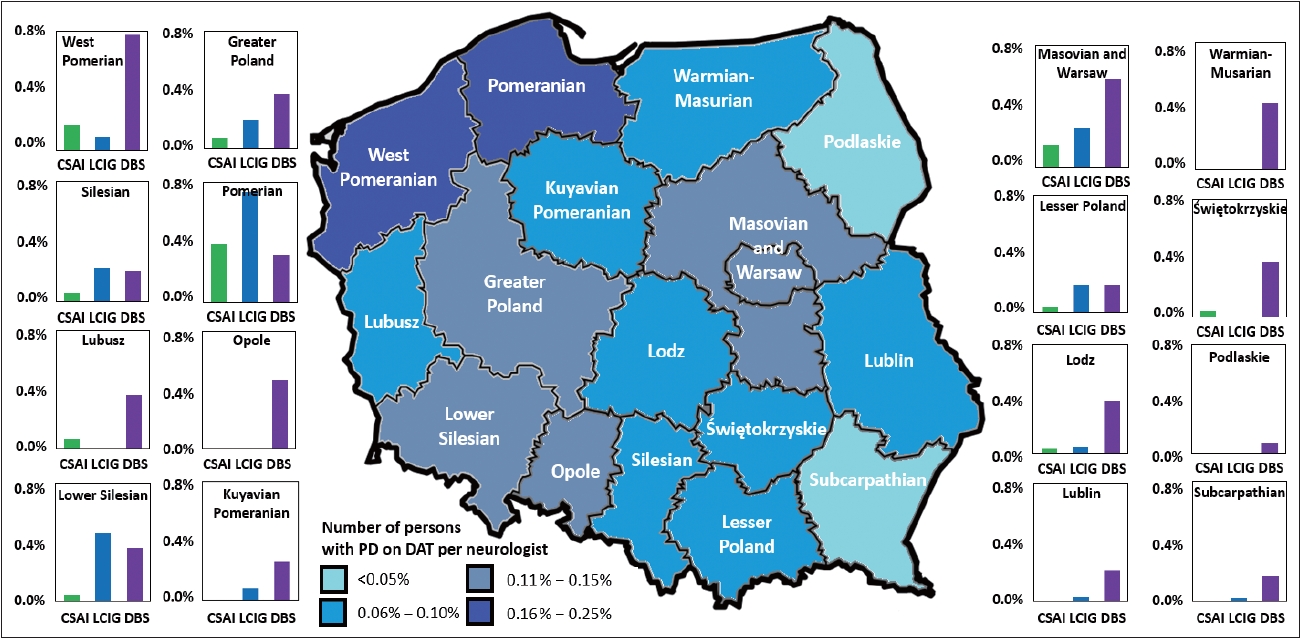
- Although overall, there were no differences in the number of persons with PD per neurologist or the number of persons with PD on DAT per neurologist across regions (p = 0.451 for both), post hoc analyses revealed that, in 2021, both the Podlaskie and Subcarpathia regions had significantly fewer persons with PD on DAT (per neurologist) compared to the Pomorskie and West Pomerania regions, which had the highest number of patients, (p ≤ 0.037) (Supplementary Table 1 in the online-only Data Supplement). In addition, we noted regional differences in the use of the specific type of DAT; in the Łódź region, DBS was the preferred DAT method (the ratio of use between CSAI, LCIG, and DBS was 1:1.5:10.5), whereas in the Lower Silesia region, both LCIG and DBS were preferred over CSAI (ratio of 1:11:8.5). The regions where the use of DAT was most evenly spread across the available options were the Pomorskie (ratio of 1:1.9:0.8) and Silesia regions (ratio of 1:3.8:3.5) (Figure 2).
- Finally, we performed univariate analyses between the number of persons with PD on DATs per neurologist and education and average annual income levels per person with PD. These analyses showed a strong positive association between annual income and the number of persons with PD on DATs per neurologist in 2021 (r = 0.799; p < 0.001) (Supplementary Figure 1 in the online-only Data Supplement) but not in 2019 (r = -0.451; p = 0.08) or for any of the years or the percentage of people who had completed higher education (p ≥ 0.053).
RESULTS
- Our results show that DAT were increasingly used in Poland during 2015–2021, showing a preference for DBS as DAT of first choice but also a steady increase in the use of CSAI and LCIG. This was also observed at a regional level, although regional differences in the proportion of use of each type of DAT were observed, similar to regional differences in other countries [10]. Part of this regional variation might be related to annual income, with regions where the annual income is higher performing more DAT procedures.
- Although we observed a steady increase in the number of people with PD for whom DAT was initiated, currently, less than 1% of persons with PD in Poland are on DAT, considerably less than in other countries [11-13]. It is estimated that 15%–30% of persons with PD should ultimately receive a form of DAT [14], but several factors can influence the actual number of people receiving DAT, which could be a reflection of conservative patterns of healthcare practices [15]. Alternatively, it might be argued that these low numbers reflect the lack of awareness and education regarding DAT in people with PD [16]. People with PD should ideally be given insight and access to all different modalities using a shared decision process [17,18].
- According to newly proposed guidelines on DATs, DBS could be recommended at a relatively early stage of PD, as soon as fluctuations are present, as this intervention can significantly improve quality of life and reduce fluctuations [19]. However, timely intervention may be hampered by waiting lists; nonetheless, data from 2023 showed that 19 out of 23 centers in Poland offer DBS within 1–7 weeks, three centers offer DBS within 7 to 12 months, and only one center offers DBS within 3 years [20]. Infusion therapies, on the other hand, are less widespread in Poland; in 2017, only two centers offered infusion therapies, increasing to nine in 2018, and 17 centers currently offer these therapies [20]. In view of the cost-effectiveness of DAT therapies [10], this is an encouraging development, especially taking into account the recommended use of DAT [14].
- The barriers to and the possible reasons for underutilization of DAT in Poland remain unclear, but it can be speculated that several factors may be involved: 1) the relatively high initial costs of DAT [6]; 2) the limited availability of DATs; 3) the relatively low number of specialized movement disorder neurologists [10]; 4) a conservative approach to PD treatment in general [15]; 5) the low number of referrals by general neurologists and general practitioners due to limited knowledge about DAT [21,22]; and 6) limited knowledge about DAT among persons with PD [23].
- While using a national registry helps minimize selection bias, there are still some limitations to consider. In the case of the NHF, patients who received fully covered procedures through private health insurance were not included, potentially causing an underestimation of the number of persons with PD on DAT in Poland. Regional variations can be influenced by specific centers’ preferences for DAT, driven by personal experiences. Patient choices of centers, possibly outside their local area, could also impact regional data, but we had no access to these data. Additionally, limitations in available databases, such as missing data on disease duration, severity, and the number of movement disorder neurologists per region, may have constrained the interpretation of our findings.
- In summary, we highlighted the increasing role of DATs in the treatment of persons with PD and demonstrated the increasing use of this treatment modality in Poland. Further efforts should be undertaken to optimize the care for persons with PD in Poland, including promoting more widespread use of DAT and addressing current barriers for their use.
DISCUSSION
Supplementary Materials
Supplementary Table 1.
Supplementary Figure 1.
-
Ethics Statement
Because this work was based on anonymized data from National Health Fund in Poland database, no approval from an institutional review board was necessary. Similarly, informed patient consent was not necessary for this work. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
-
Availability of Data and Material
The data supporting the findings of this study are available within the article and/or its supplementary material.
-
Conflicts of Interest
KS has received a travel grant and speaker fees from AbbVie. TP no relevant financial disclosures. KRC has received funding from Parkinson’s UK, NIHR, UCB, and the European Union; he received honoraria from UCB, Abbott, Britannia, US Worldmeds, and Otsuka Pharmaceuticals; and acted as a consultant for AbbVie, UCB, and Britannia. BRB is co-Editor in Chief for the Journal of Parkinson’s Disease. He is on the editorial board of Practical Neurology and Digital Biomarkers, has received honoraria from being on the scientific advisory board for AbbVie, Biogen, and UCB, has received fees for speaking at conferences from AbbVie, Zambon, Roche, GE Healthcare, and Bial, and has received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, UCB, Not Impossible, the Hersenstichting Nederland, the Parkinson’s Foundation, Verily Life Sciences, Horizon 2020, and the Parkinson Vereniging (all paid to the institute). DvW has received a travel grant and speaker fees from Bial, as well as speaker fees from Britannia Pharmaceuticals, and is supported by a research grant from CHDI.
-
Funding Statement
None
-
Author Contributions
Conceptualization: Katarzyna Smilowska, Daniel J. van Wamelen. Formal analysis: Katarzyna Smilowska, Daniel J. van Wamelen. Investigation: Katarzyna Smilowska, Daniel J. van Wamelen. Methodology: Katarzyna Smilowska, Daniel J. van Wamelen. Project administration: Katarzyna Smilowska, Daniel J. van Wamelen. Resources: Katarzyna Smilowska, Daniel J. van Wamelen, Tomasz Pietrzykowski. Software: Daniel J. van Wamelen. Supervision: Daniel J. van Wamelen, Tomasz Pietrzykowski, K. Ray Chaudhuri, Bastiaan R. Bloem. Visualization: Katarzyna Smilowska, Daniel J. van Wamelen, Bastiaan R. Bloem. Writing—original draft: Katarzyna Smilowska, Daniel J. van Wamelen. Writing—review & editing: Tomasz Pietrzykowski, K. Ray Chaudhuri, Bastiaan R. Bloem.
Notes
- We thank Beata Kon for assistance that contributed to the development of this paper.
- The opinions expressed in this publication are those of the authors/researchers, and do not necessarily reflect the official views of the National Health Fond of Poland.
Acknowledgments

- 1. Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 2011;26(Suppl 1):S1–S58.ArticlePubMedPDF
- 2. Antonini A, Moro E, Godeiro C, Reichmann H. Medical and surgical management of advanced Parkinson’s disease. Mov Disord 2018;33:900–908.ArticlePubMedPDF
- 3. Storch A, Schneider CB, Wolz M, Stürwald Y, Nebe A, Odin P, et al. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 2013;80:800–809.ArticlePubMed
- 4. van Wamelen DJ, Grigoriou S, Chaudhuri KR, Odin P. Continuous drug delivery aiming continuous dopaminergic stimulation in Parkinson’s disease. J Parkinsons Dis 2018;8(S1):S65–S72.ArticlePubMedPMC
- 5. Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin 2018;34:2063–2073.ArticlePubMed
- 6. Smilowska K, van Wamelen DJ, Pietrzykowski T, Calvano A, RodriguezBlazquez C, Martinez-Martin P, et al. Cost-effectiveness of device-aided therapies in Parkinson’s disease: a structured review. J Parkinsons Dis 2021;11:475–489.ArticlePubMedPMC
- 7. Lökk J. Lack of information and access to advanced treatment for Parkinson’s disease patients. J Multidiscip Healthc 2011;4:433–439.PubMedPMC
- 8. Narodowy Fundusz Zdrowia. National Health Fund [Internet]. Warszawa: Narodowy Fundusz Zdrowia [accessed on 2023 May 8]. Available at: https://www.nfz.gov.pl.
- 9. Statistics Poland. Warszawa: Statistics Poland [Internet]. Warszawa: Statistics Poland [accessed on 2023 Apr 23]. Available at: https://stat.gov.pl/.
- 10. Henriksen T, Dalhoff KP, Hansen HE, Brenneche AW, Lønberg US, Danielsen EH. Access and use of device-aided therapies for Parkinson’s disease in Denmark. Mov Disord Clin Pract 2020;7:656–663.PubMedPMC
- 11. Pilitsis JG, Burrows A, Peters ML, Sargent J, Ng SC, Tseng JF. Changing practice patterns of deep brain stimulation in Parkinson’s disease and essential tremor in the USA. Stereotact Funct Neurosurg 2012;90:25–29.ArticlePubMedPDF
- 12. Lad SP, Kalanithi PS, Patil CG, Itthimathin P, Batya S, Bronte-Stewart H, et al. Socioeconomic trends in deep brain stimulation (DBS) surgery. Neuromodulation 2010;13:182–186.ArticlePubMed
- 13. Poortvliet PC, Silburn PA, Coyne TJ, Chenery HJ. Deep brain stimulation for Parkinson disease in Australia: current scientific and clinical status. Intern Med J 2015;45:134–139.PubMed
- 14. Norlin JM, Willis M, Persson U, Andersson E, E Pålhagen S, Odin P. Swedish guidelines for device-aided therapies in Parkinson’s disease—Economic evaluation and implementation. Acta Neurol Scand 2021;144:170–178.ArticlePubMedPDF
- 15. Christen M, Müller S. Current status and future challenges of deep brain stimulation in Switzerland. Swiss Med Wkly 2012;142:w13570.ArticlePubMed
- 16. De Pandis MF, Torti M, Rotondo R, Iodice L, Levi Della Vida M, Casali M, et al. Therapeutic education for empowerment and engagement in patients with Parkinson’s disease: a non-pharmacological, interventional, multicentric, randomized controlled trial. Front Neurol 2023;14:1167685.ArticlePubMedPMC
- 17. Nijhuis FAP, Esselink R, de Bie RMA, Groenewoud H, Bloem BR, Post B, et al. Translating evidence to advanced Parkinson’s disease patients: a systematic review and meta-analysis. Mov Disord 2021;36:1293–1307.ArticlePubMedPMCPDF
- 18. Nijhuis FA, van Heek J, Bloem BR, Post B, Faber MJ. Choosing an advanced therapy in Parkinson’s disease; is it an evidence-based decision in current practice? J Parkinsons Dis 2016;6:533–543.ArticlePubMed
- 19. Deuschl G, Antonini A, Costa J, Śmiłowska K, Berg D, Corvol JC, et al. European Academy of Neurology/Movement Disorder Society-European section guideline on the treatment of Parkinson’s disease: I. Invasive therapies. Mov Disord 2022;37:1360–1374.ArticlePubMedPDF
- 20. Narodowy Fundusz Zdrowia. Information on treatment availability [Internet]. Warszawa: Narodowy Fundusz Zdrowia [accessed on 2023 Apr 23]. Available at: https://terminyleczenia.nfz.gov.pl/.
- 21. Cabrera LY, Young Han C, Ostendorf T, Jimenez-Shahed J, Sarva H. Neurologists’ attitudes toward use and timing of deep brain stimulation. Neurol Clin Pract 2021;11:506–516.ArticlePubMedPMC
- 22. Fujioka S, Mishima T, Yamazaki T, Bebrysz M, Nomoto M, Yamaguchi J, et al. Neurologists’ preferences for device-aided therapy for advanced Parkinson’s disease in Japan. Curr Med Res Opin 2023;39:91–104.ArticlePubMed
- 23. Tan AH, Tan CT, Marras C, Loh KW, Wye Ho NW, Lim QH, et al. Knowledge of Parkinson’s disease in a multiethnic urban Asian setting. J Parkinsons Dis 2015;5:865–879.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

Comments on this article
- Figure
- Related articles
-
- A Survey of Perspectives on Telemedicine for Patients With Parkinson’s Disease
- Current Status and Future Perspectives on Stem Cell-Based Therapies for Parkinson’s Disease
- Umami and Other Taste Perceptions in Patients With Parkinson’s Disease
- Association of AXIN1 With Parkinson’s Disease in a Taiwanese Population
- Resilience and Trauma among Patients with Parkinson’s Disease during the COVID-19 Pandemic
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite