Articles
- Page Path
- HOME > J Mov Disord > Volume 17(1); 2024 > Article
-
Original Article
Analysis of Semiology, Lesion Topography and Treatment Outcomes: A Prospective Study on Post Thalamic Stroke Holmes Tremor -
Amlan Kusum Datta1
 , Adreesh Mukherjee1
, Adreesh Mukherjee1 , Sudeshna Malakar2
, Sudeshna Malakar2 , Atanu Biswas1
, Atanu Biswas1

-
Journal of Movement Disorders 2024;17(1):71-81.
DOI: https://doi.org/10.14802/jmd.23095
Published online: October 20, 2023
1Institute of Post Graduate Medical Education & Research and Bangur Institute of Neurosciences, West Bengal, India
2Department of Radiology, Apollo Multispeciality Hospitals, West Bengal, India
- Corresponding author: Atanu Biswas, MD, DM, FIAN Institute of Post Graduate Medical Education & Research and Bangur Institute of Neurosciences, 52/1A Sambhunath Pandit Street, Bhowanipore, Kolkata, West Bengal 700025, India / Tel: +91-9836368139 / Fax: +91-33 2223 6677 / E-mail: atabis@gmail.com
Copyright © 2024 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,137 Views
- 100 Download
ABSTRACT
-
Objective
- Holmes tremor (HT) comprises rest, postural and intention tremor subtypes, usually involving both proximal and distal musculature. Perturbations of nigro-striatal pathways might be fundamental in the pathogenesis of HT along with cerebello-thalamic connections.
-
Methods
- Nine patients with an HT phenotype secondary to thalamic stroke were included. Epidemiological and clinical records were obtained. Structural and functional brain imaging were performed with magnetic resonance imaging (MRI) or computed tomography (CT) and positron emission tomography (PET), respectively. Levodopa was administered in sequentially increasing dosage, with various other drugs in case of inadequate response. Longitudinal follow-up was performed for at least three months. The essential tremor rating assessment scale (TETRAS) was used for assessment.
-
Results
- The mean latency from stroke to tremor onset was 50.4 ± 30.60 days (range 21–90 days). Dystonia was the most frequently associated hyperkinetic movement (88.8%). Tremor was bilateral in 22.2% of participants. Clinical response was judged based on a reduction in the TETRAS score by a prefixed value (≥ 30%), pertaining to which 55.5% (n = 5) of subjects were classified as responders and the rest as non-responders. The responders showed improvement with significantly lower doses of levodopa than the remaining nonresponders (240 ± 54.7 mg vs. 400 ± 40.8 mg; p = 0.012).
-
Conclusion
- Although levodopa is useful in HT, augmenting the dosage of levodopa beyond a certain point might not benefit patients clinically. Topography of vascular lesions within the thalamus might additionally influence the phenomenology of HT.
- More than a century after the original description by Gordon Holmes, the pathophysiology of Holmes tremor (HT) remains an enigma. Previous nomenclature such as “rubral tremor”, “thalamic tremor”, and “mesencephalic tremor” have been largely abandoned owing to numerous studies that have elucidated the network of various regions in the brain, which might contribute to the genesis of HT [1-3]. This “network hypothesis” proposes the concurrent functional defect of both cerebello-thalamic or dentato-rubral-thalamic and dopaminergic nigrostriatal pathways as requisite for the genesis of such a tremor [3,4]. The most recent consensus is to consider HT as a clinical syndrome that comprises rest, postural and intention tremor subtypes, usually involving both proximal and distal musculature, at a low frequency (< 5 Hz) [5].
- Vascular lesions are by far the most common etiological cause of HT, with a variable latency from initial insult to onset of tremor ranging from as low as one week to more than a decade [3,6]. This thereby underscores the role of neuronal plasticity and the reorganization of different neuronal circuits within proposed brain regions, the intricacies of which are presently beyond our understanding. Irrespective of the etiology, however, the therapeutic response to pharmacological agents, including dopaminergic medication, has been heterogeneous [7]. In contrast, the response to deep brain stimulation (DBS) surgery has been more uniform and encouraging [2,8].
- In concurrence with Nsengiyumva et al. [6], we believe that the anatomical localization of the HT lesion harbors significance with regard to its therapeutic prospects. Herein, we elucidate a series of patients who presented with HT, with variable latency following a thalamic vascular insult, as well as look at the natural history of such lesions and therapeutic response on follow-up.
INTRODUCTION
- The investigators included patients from the outpatient department (OPD) of a tertiary care neurology institute in Kolkata, India, between July 2021 and June 2022. A total of approximately 230 patients presenting with tremor were screened in the movement disorder clinic within the stipulated period, of which 11 patients with the HT phenotype were identified (4.78%). One patient was excluded because brain imaging could not be performed and was lost to follow-up. Nine patients had thalamic lesions, while one showed a brainstem lesion. For the present study, only patients with thalamic lesions were included. The diagnosis of HT was clinical, based on the definition in the Consensus statement of the International Parkinson disease and Movement disorder society [5].
- All the patients were critically examined, and their findings were analyzed by three neurologists (AKD, AM and AB), two of whom (AM and AB) are movement disorder specialists at the same institute. All patients were followed up for at least three months after the initial evaluation and start of therapy. The following data were collected: age, sex, latency from initial insult to onset of tremor, neurological and radiological features, therapy provided, and response.
- All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee of Institute of Post Graduate Medical Education & Research (IPGME & R), Kolkata (IPGME&R/IEC/2023/290) and with the 1975 Helsinki declaration and its later amendments. Written informed consent was duly obtained from the patients included in the study. This included consent to 1) participate in the study, 2) for video-recording, and 3) presentation or publication of videos for academic purpose.
- Treatment of all patients was started within one week of presentation to the movement disorder clinic. Tremor severity was quantified as per the essential tremor rating assessment scale (TETRAS). Patients were treated with sequentially increasing doses of levodopa with other add-on drugs as necessitated by response and tolerability. Patients were classified into responders and nonresponders based on a predetermined cutoff reduction in the performance score (PS) subset of TETRAS by 30% or more. The longitudinal follow-up duration was at least three months, and patients were independently assessed by all three investigators in the same clinical setting using TETRAS; however, a common consensus was agreed upon by all the investigators regarding initial diagnosis and outcomes.
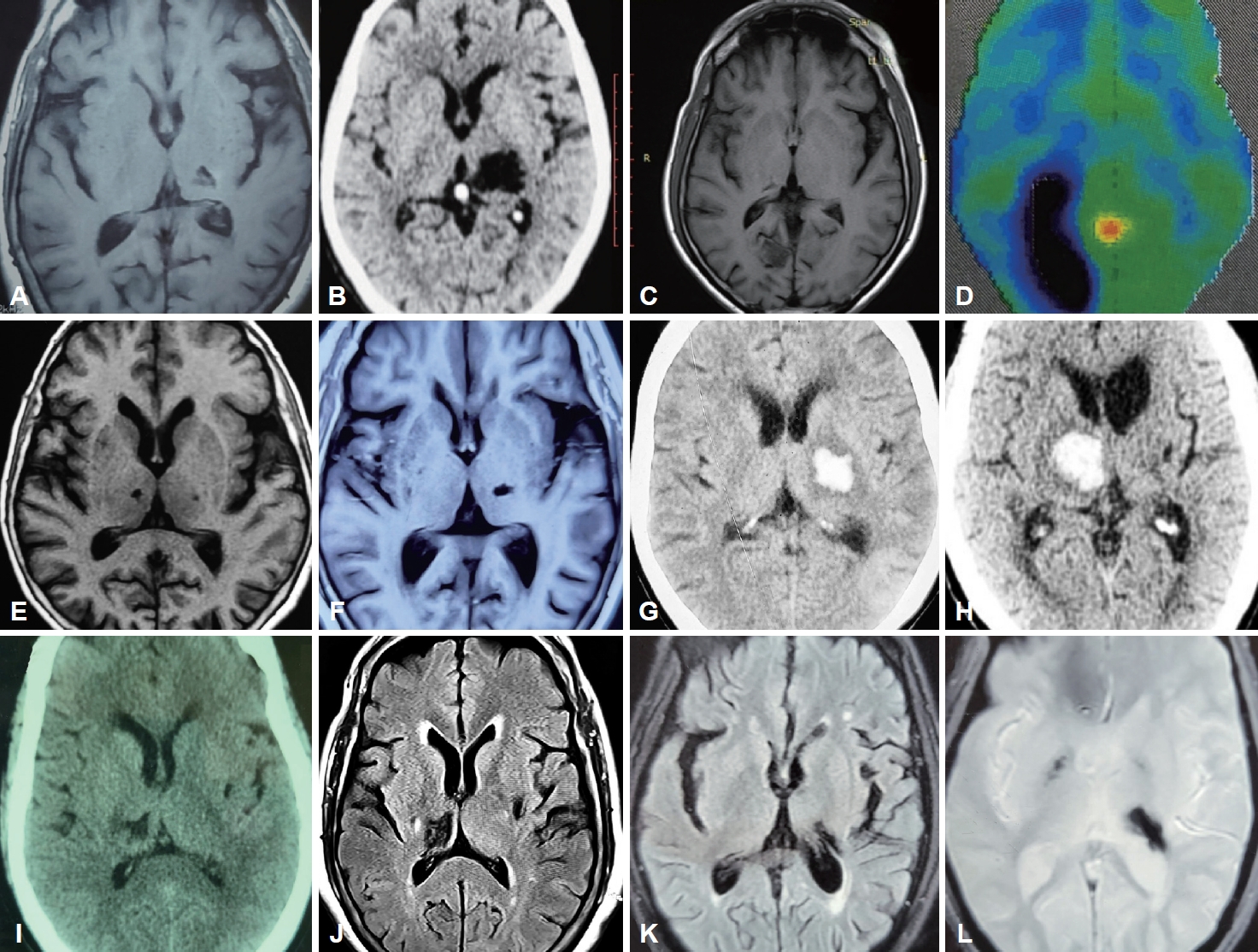
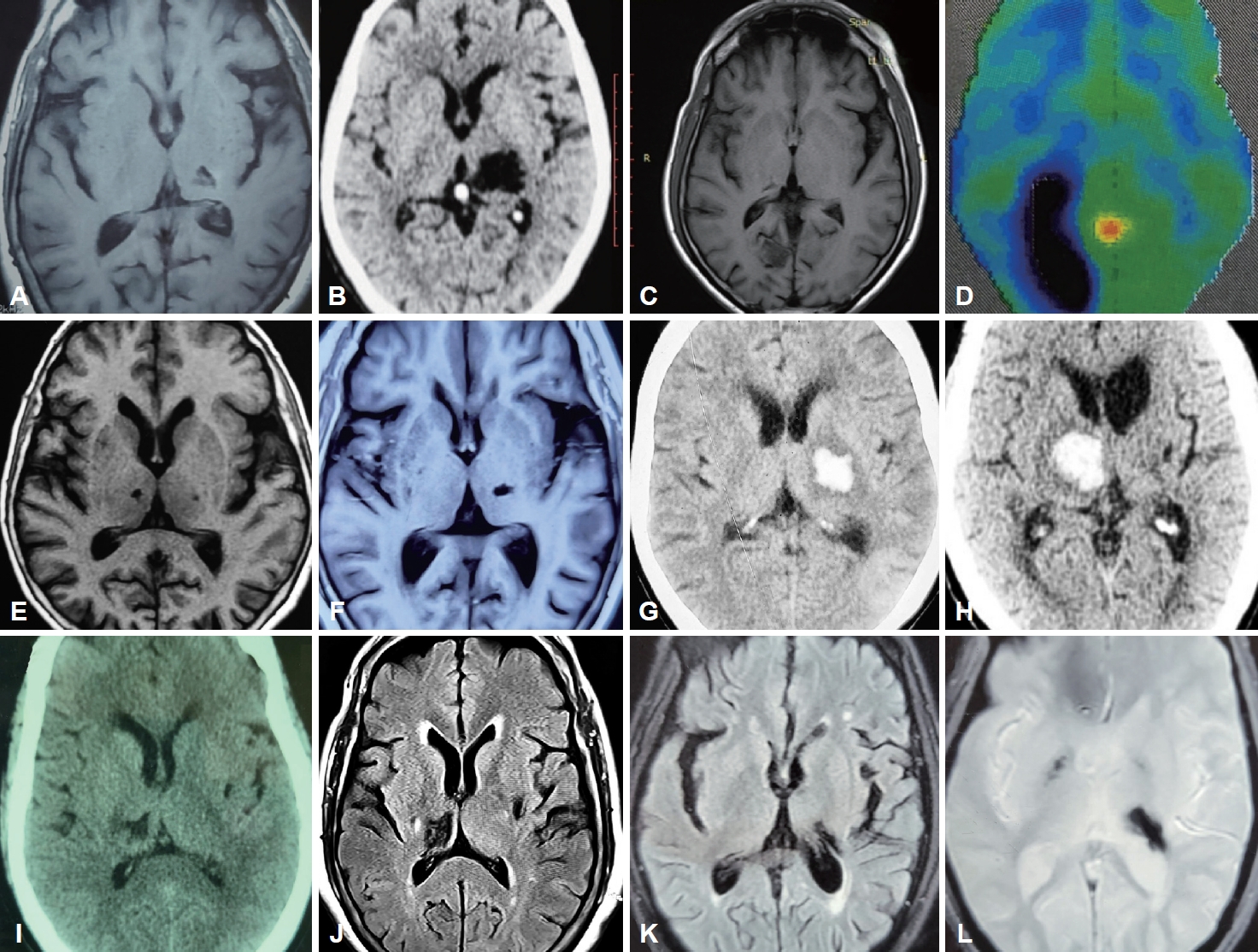
- Brain imaging was performed with either magnetic resonance imaging (MRI) using a 3 Tesla scanner or computed tomography (CT) using a 128-slice machine. Functional imaging was performed with a fluorodeoxyglucose positron emission tomography (FDG-PET) scan. MRI sequences used for evaluation were axial, coronal, and sagittal T1, T2 weighted images, axial fluid attenuated inversion recovery (FLAIR) sequence, axial diffusion weighted image (DWI), axial apparent diffusion coefficient (ADC) maps, axial gradient echo (GRE) sequence and time of flight (TOF) angiography images of cerebral vasculature. Images were assessed independently by a neuroradiologist (S.M.) who was blinded to the clinical features of each patient.
- Data were organized in Microsoft Excel (Microsoft, Redmond, WA, USA) and analyzed in IBM SPSS v.23 (IBM Corp., Armonk, NY, USA). Categorical variables are expressed as frequencies and percentages. Continuous variables are expressed as the mean and standard deviation. Comparisons between groups were performed using the Mann‒Whitney U test after checking for assumptions.
MATERIALS & METHODS
- Patient demographics
- Nine patients were included for analysis, all of whom were of Bengali ethnicity. The cohort comprised 4 male and 5 female patients. The mean age of presentation was 53.3 ± 8.4 years (range 40–70 years). The mean latency from initial vascular insult to onset of tremor was approximately 50.4 ± 30.60 days (range: 21–90 days) (Table 1).
- Etiology and radiological features
- Fifty-five percent of the patients (n = 5) presented following ischemic strokes, while the remaining patients had sequelae of hemorrhagic stroke, all involving the thalamus. Among the ischemic thalamic lesions (n = 5), thalamoperforating and/or posterior choroidal circulation was implicated in n = 3 (60%); the remaining patients (n = 2; 40%) had affection of the thalamogeniculate vasculature. Involvement of the occipital lobe and putamen was noted in one patient each (Figure 1).
- Clinical features
- Dystonia was the most frequently associated hyperkinetic movement of the affected limb (n = 8; 88.8%). Frontalis and cervical dystonia were noted in one patient each (n = 1; 11.1%). A single patient had voice tremor (n = 1; 11.1%), while another had dysarthria (n = 1; 11.1%). Chorea was a feature in one patient (n = 1;11.1%). The tremor was bilateral in two patients (n = 2; 22.2%), while lower limb tremor was prominent in a single patient (n = 1; 11.1%). Tremor was noted to be distal predominant in n = 4; 44.4% of patients. Hemiparesis/monoparesis was the most commonly associated neurodeficit (n = 8; 88.8%), followed by proprioceptive defects of the ipsilateral limb (n = 4; 44.4%) and hemihypesthesia (n = 2; 22.2%). Two patients had cranial nerve deficits (n = 2; 22.2%). Depression/psychosomatic symptoms were noted in two participants (n = 2; 22.2%) (Tables 1, 2).
- Treatment outcomes
- All patients were managed with pharmacological therapy, which included sequentially increasing doses of levodopa, followed by various combinations of trihexyphenidyl, clonazepam, baclofen and levetiracetam (Table 2). The mean dosage of levodopa administered was 311.11 ± 96.1 mg (range: 200–450 mg). All patients were followed up for at least 3 months following the onset of therapy. The patients were classified into responders and nonresponders as per preset criteria (30% or more reduction in performance score of TETRAS score). Five subjects (55.5%) were deemed to be responders after independent assessment by all three investigators at 3 months or beyond of follow-up, while the remaining (n = 4; 44.4%) were categorized as nonresponders. The mean performance score (PS) score of TETRAS in responders prior to therapy was 19.0 ± 3.5, which was significantly lower after therapy at 9.6 ± 1.1 (p = 0.014). The mean levodopa dose in responders was 240 ± 54.7 mg, which was significantly lower than the levodopa dosage in nonresponders (400 ± 40.8 mg; p = 0.012) (Table 3).
RESULTS
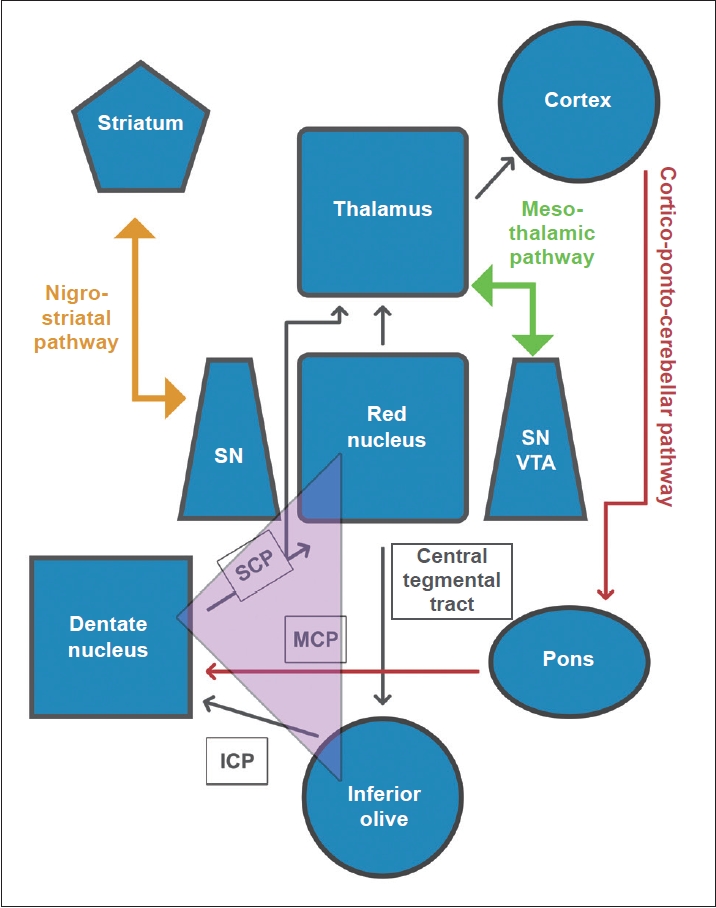
- It is now largely accepted that HT is a heterogeneous clinical entity, with significant clinical variations both in its semiology and etiology, a fact that was acknowledged by Gordon Holmes himself more than a century ago [6,9]. Holmes, in his seminal work, attributed the classic tremor to lesions of the red nucleus or the cerebello-rubral system, emphasizing that the former is phylogenetically a part of the thalamencephalon. The phenomenon that a thalamic vascular insult can give rise to a high-amplitude, low-frequency tremor with all three components is well documented [10-12]; this observation is in congruence with the putative role of the thalamus in the so-called “long loop”, i.e., the dentate-rubro-thalamo-cortico-pontine-cerebellar pathway [13]. Although thalamic lesions have been shown to account for several new-onset HT phenotypes [3], various other anatomical locations have also been similarly implicated in its genesis; these include the red nucleus, globus pallidus (GP), ponto-mesencephalic junction, cerebellar cortex, and vermis [2]. The involvement of the nigro-striatal pathway, however, is speculative and is derived mostly from the observation that HT is responsive to levodopa and the asymmetry of uptake demonstrated in functional dopaminergic studies [2,3,14].
- Thalamus and Holmes’ tremor
- The authors described nine cases that presented with an HT phenotype with or without other associated hyperkinetic movements, with variable latencies following a thalamic vascular insult. The relative paucity of resting tremors in some of these patients is in accordance with previous observations that thalamic lesions are unlikely to generate resting state tremors, unless particularly severe [10,11,15-17]. According to a plausible hypothesis, synchronized oscillations of the inferior olive (IO) nucleus, because of perturbations of the so-called “short loop”, i.e., dentatoolivary-cerebellar pathway, are believed to cause regular, involuntary tremors such as static and rest tremors [13]. In HT, nigrostriatal pathways are often implicated along with cerebello-thalamic tracts, and the former might contribute to the generation of the restrest tremor. According to the recent lesion connectome concept in HT, the involved thalamic nuclei are the ventralis oralis posterior (VOP) and pulvinar [1] rather than the ventralis intermediate (VIM), which is commonly targeted in DBS for tremor. Furthermore, a study using multimodal 3D medical imaging in HT revealed a differing clinical picture and therapeutic response in HT depending on the involved thalamic nuclei and their connections [18]. Hence, clinical phenotyping could be pivotal in the selection of appropriate target(s) for DBS in refractory HT.
- Among the patients with thalamic infarctions (patients 1, 3, 4, 5 and 8), the vascular territory was thalamo-geniculate with involvement of the lateral part of the thalamus in two patients (patients 1 and 4), while the posterior choroidal artery/thalamoperforate territories were implicated in three subjects (patients 3, 5 and 8). This is in coherence with works of previous authors [10,12,15] who had implicated the thalamogeniculate and, in few cases, the posterior choroidal territories in the pathogenesis of involuntary abnormal movements following thalamic vascular insult. It is worth noting that involvement of the ventrolateral thalamic nuclei, particularly the VIM nucleus, is fundamental to the genesis of tremor and hyperkinetic movements, as it is an integral part of the cerebello-thalamo-cortical loop [19]. Whether the interruption occurs at the level of the VIM or prior to entry of the cerebello-thalamic fibers into the nucleus, however, is unknown, since it has been shown that the VIM lies outside the brain circuit responsible for HT [1]. In fact, the lesion connectome concept in HT affects the VOP and pulvinar nuclei as culprits for tremor genesis. Previously, Krystkowiak et al. [10] and Lehéricy et al. [12] suggested lesions of the centromedian (Cm) thalamic nucleus as a cause of dystonia; however, more intricate networks might be at play.
- The authors noted that both the tremor and associated dystonia improved with levodopa therapy, which might be due to the functional proximity of the paramedian thalamic territories to the mesencephalon and the nigro-striatal tract (Figure 2). The concept of a direct connection between the mesencephalic dopaminergic system and the paramedian thalamic nuclei groups, i.e., the “mesothalamic pathway”, was proposed by Freeman et al. [20]. Lesions of this pathway are believed to cause retrograde degeneration of the upstream basal ganglia and nigrostriatum. A recent meta-analysis underscores levodopa as the most effective pharmacological agent for HT [21], thus strengthening previous hypotheses emphasizing the role of nigrostriatal dopaminergic denervation in the pathogenesis of HT. However, this pathway alone might not be enough to explain all aspects of the HT phenotype.
- Dystonia in HT
- Dystonia was the most commonly associated hyperkinetic movement, and it was present in the majority of patients (8/9; 88.8%). The distribution of dystonia mostly followed the tremor-affected limb, although it also involved the lower limbs in 2 patients. An irregular, jerky, dystonic head, and neck tremor was a feature of one of the patients in the series (patient 2). Bilateral involvement of the upper limbs was noted in two of the subjects (patients 5 and 8). It has been previously described that movement disorders resulting from thalamic lesions are mostly mixed phenomena [15,17]. Dystonia was present in nearly 83% of cases in a recent study on HT (Table 1) [22]. The authors found that dystonia was ameliorated following levodopa therapy, which might implicate pallido-thalamic and nigro-striatal pathways in the pathogenesis of dystonia in HT.
- Chorea in HT
- Another interesting observation in the present series is that one patient (patient 1) had initially developed choreiform movement of the affected extremity, which was coupled with dystonic posturing. Chorea is an exceedingly rare manifestation of thalamic stroke. Acute pure hemichorea has been previously reported in thalamic lacunar infarction [23]; however, the patient in the present study had a delayed onset by a few weeks. This might be attributable to perturbations in thalamostriatal connections and cerebral plasticity.
- Functional imaging, role of FDG-PET and abnormal sensory feedback
- Recovery in motor function is linked to synaptic plasticity and reorganization of motor tracts in the frontal motor and premotor cortex as well as recruitment of uncrossed pyramidal fibers from the opposite motor cortex and contralateral cerebellum [24]. Hence, it is appealing to consider that these newly formed pyramidal motor/cerebellar pathways might be unstable and misdirected, with perturbed sensory-motor feedback and feed-forward mechanisms, leading to the development of tremors in the affected limb. The authors found hypometabolism on brain FDG-PET in the bilateral inferior frontal cortex in one patient (patient 3) who had ischemic stroke involving the right occipital cortex and medial thalamus. Functional aberrations of thalamo-striato-cortical connections might have contributed to decreased metabolism in areas that are otherwise anatomically discrete from the primary site of vascular insult.
- Presently, there is increasing evidence to support the role of abnormal sensory motor integration in the pathogenesis of many movement disorders. In this regard, proprioceptive afferent impulses are particularly important for smooth coordination and execution of movements [25]. The basal ganglia have numerous reciprocal connections with the frontal and limbic cortex and the thalamic nuclei, which in turn are regulated by dopamine and dopamine receptors. These connections comprise two distinct loops, namely, the direct and indirect pathways, which exert facilitatory and inhibitory influences on the motor cortex, respectively. Successful execution of motor control, however, is critically linked to appropriate sensory feedback; therefore, the basal ganglia serve as a gateway to sensory feedback for motor control [26]. The cerebellum directly receives rich sensory information, and this plays an important role in motor coordination [27]. Thus, the authors hypothesize that disrupted central processing of sensory information might play a significant role in the genesis of hyperkinetic movements such as tremor, which might additionally reduce the efficacy of treatment modalities that mostly focus on motor circuitry.
- Treatment of HT
- HT is a source of major debilitation to the patient, owing to its large amplitude and refractoriness to conventional pharmacotherapy. Efforts have been made to treat it with several agents, namely, levodopa and anticholinergics; however, outcomes have been variable and largely unsatisfactory [2,3]. Surgical options such as DBS have achieved considerable success in ameliorating all tremor subtypes, including HT [28]. However, the most common therapeutic target for HT DBS therapy, the VIM, has been associated with remission in less than half of such cases [1]. Therefore, in the future, it may be beneficial to shift the focus to other targets, such as the GP, subthalamic nucleus (STN), and other thalamic nuclei (such as the VOP), which are more integral parts of the HT circuit [1]. In the present study, the mean dosage of levodopa used to achieve definitive clinical response was significantly lower in the responder cohort. The authors observed that increasing the daily dosage of levodopa beyond 300 mg/day does not add much clinical benefit; in contrast, it causes various side effects (nausea, vomiting, dizziness) and tolerability issues. What exactly should be the cutoff daily dosage beyond which therapeutic benefit is unlikely is a question that needs to be addressed through future controlled prospective studies in larger cohorts of patients.
- Dopamine transporter scan and appraisal of the role of the nigrostriatal pathway
- Likewise, the role of the nigrostriatal pathway in the pathogenesis of HT has been a subject of contention for decades. Several researchers have attempted to evaluate cases of HT of various etiologies with functional dopamine uptake scans [2,4,7,29-36] (Table 4). Remy et al. [7] were the first to examine functional aberrations at both the pre- and postsynaptic dopaminergic levels and demonstrated significant presynaptic reductions in dopamine uptake as well as a response to dopaminergic therapy. Similar observations of reduced or absent presynaptic dopamine uptake and dopaminergic therapy response were reported by Strecker et al. [31], Guedj et al. [32], Seidel et al. [4], and Juri et al. [35]. However, this is debatable since other authors have reported contrary observations. Sung et al. [33] and Paviour et al. [29] failed to document a dopaminergic therapeutic response despite a reduction in striatal presynaptic dopamine uptake. Hertel et al. [30] and Gajos et al. [2] reported cases of midbrain and thalamic HT, respectively, with normal uptake in pre- and postsynaptic domains (Table 4). Considering the inconsistencies in these studies, it would be premature to assume that the involvement of nigrostriatal is sine qua non for the genesis of HT. Despite these controversies, the role of levodopa in the treatment of HT remains undeniable and should be part of a therapeutic trial of all such patients.
- The present study has a few limitations. First, the number of participants was small, and no statistically significant conclusion can be drawn based on the present findings. Second, functional brain imaging was not used in the evaluation of every patient due to various contraindications and lack of logistic support; hence, the assumptions are chiefly based on clinical and structural radiological findings and clinical outcomes, leaving scope for investigator bias. Third, the TETRAS scale used for quantifying tremor severity was originally meant for essential tremor phenotypes and does not take into consideration the remaining component of tremor. However, the patients were independently assessed by movement disorder specialists with concurrence of opinion regarding improvement.
- In hindsight, thalamic lesions led to distinct, heterogeneous tremor phenotypes with variable responses to therapy. Dystonia was frequently associated with tremor. The phenotype of HT was not uniform, and it varied according to the localization of the lesion within the thalamus. The tremor improved with levodopa in the majority of the patients. An increase in the daily dosage of levodopa beyond a certain point did not add any clinical benefit, and those who responded did so at significantly lower doses than those refractory to treatment.
DISCUSSION
Supplementary Material
Video 1.
Video 2.
Video 3.
Video 4.
Video 5.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None
-
Author contributions
Conceptualization: Amlan Kusum Datta, Adreesh Mukherjee. Data curation: Amlan Kusum Datta, Adreesh Mukherjee. Formal analysis: all authors. Investigation: Amlan Kusum Datta, Adreesh Mukherjee, Sudeshna Malakar. Methodology: Amlan Kusum Datta, Adreesh Mukherjee, Atanu Biswas. Project administration: Atanu Biswas, Amlan Kusum Datta. Resources: Amlan Kusum Datta, Adreesh Mukherjee, Atanu Biswas. Software: Amlan Kusum Datta, Adreesh Mukherjee, Atanu Biswas. Supervision: Atanu Biswas, Adreesh Mukherjee. Validation: Sudeshna Malakar, Adreesh Mukherjee, Atanu Biswas. Visualization: Amlan Kusum Datta, Adreesh Mukherjee, Atanu Biswas. Writing—original draft: Amlan Kusum Datta. Writing—review & editing: Adreesh Mukherjee, Atanu Biswas, Sudeshna Malakar.
Notes
| Gajos et al., [2] 2010 | Raina et al., [3] 2016 | Nsengiyumva et al., [6] 2021 | Mishra et al., [22] 2022 | Present study | |
|---|---|---|---|---|---|
| Number of patients | 10 | 29 | 17 | 12 | 9 |
| Gender distribution (male:female) | 5:5 | 13:16 | 9:8 | 11:1 | 4:5 |
| Mean age of onset, yr | 44.8 ± 20.2 | 33.9 ± 20.1 | 45 ± 18.39 | 31.08 ± 10.1 | 53.3 ± 8.4 |
| Latency between initia insult and onset of tremor | Mean: 6.3 months (range: 1 month–2 years) | Median: 2 months (range: 7 days–228 months) | Range: 8 weeks–14 years | Mean: 4.78 months (range: 7days–1 year) | Mean: 50.4 days (range: 21–90 days) |
| Etiology | Vascular (n = 8; 80%) | Vascular (n = 14; 48.3%) | Vascular (n = 11; 64.7%) | Vascular (n = 8, 66.7%) | Vascular (100%) |
| Head trauma (n = 2; 20%) | Head trauma (n = 5; 17.2%) | Head trauma (n = 2; 11.8%) | Head trauma (n = 2, 16.7%) | ||
| Miscellaneous (n = 10; | Others (n = 4, 23.5%) | Tumor resection (n = 2, 16.7%) | |||
| Neurological deficit(s) (apart from hyperkinetic movement) | Hemiparesis (n = 5; 50%) | Hemiparesis (n = 18; 62%) | Mild hemiparesis or monoparesis (n = 5; 29.41%) | Hemiparesis (n = 7; 58.3%) | Hemiparesis (n = 6; 66.6%) |
| Cranial neuropathies (n = 7; 70%) | Ataxia (n = 15; 51.7%) | Cranial neuropathy (n = 6; 35.3%) | Ataxia (n = 7; 58.3%) | Monoparesis (n = 2; 22.2%) | |
| Ataxia (n = 3; 30%) | Hypesthesia (n = 8; 27.58%) | Ataxia (n = 5; 29.4%) | Hemi-anesthesia (n = 2; 16.7%) | Abnormal proprioception (n = 4; 44.4%) | |
| Atrophy of small muscles of hand (n = 1; 10%) | Cranial neuropathy (n = 7; 24.1%) | Proprioceptive sensory loss in affected limb (n = 4; 23.5%) | Quadriparesis (n = 2; 16.7%) | ||
| Hemi-hypesthesia (n = 1; 10%) | Dysarthria (n = 7; 24.1%) | Dysarthria (n = 2; 16.7%) | Hemi-hypesthesia (n = 2; 22.2%) | ||
| Depression (n = 1; 10%) | Vertical gaze abnormality (n = 2; 6.9%) | Dysarthria (n = 2; 11.8%) | Cognitive impairments (n = 2; 16.7%) | Cranial neuropathy (n = 2; 22.2%) | |
| Dementia (n = 1; 10%) | Seizures (n = 1; 3.4%) | Cognitive impairment (n = 2; 11.8%) | Seizures (n = 1; 8.3%) | Dysarthria (n = 1; 11.1%) | |
| Psychiatric disorders (n = 1; 3.4%) | Parinaud’s syndrome (n = 1; 5.9%) | Vertical gaze restriction (n = 1; 8.3%) | Psychiatric abnormalities (n = 2; 22.2%) | ||
| Homonymous hemianopia (n = 1; 8.3%) | |||||
| Associated movements | None reported | Dystonia (n = 7; 24.1%); 3 patients had dystonic jerks | Dystonia of affected limb (n = 6; 20.7%). | Dystonia (n = 10; 83.3%) | Dystonia (n = 8; 88.8%) |
| One patient experienced dystonic jerks “no-no” head tremor (n = 2; 11.8%) | Tongue dyskinesia (n = 1; 8.3%) | Chorea (n = 1; 11.1%) | |||
| Myoclonus (n = 1; 3.4%) | Pseudoathetosis (n = 2; 11.8%) | Tongue protrusion tremor (n = 1; 8.3%) | Dystonic head tremor (n = 1; 11.1%) | ||
| Distal choreiform movement of affected limb (n = 2; 11.8%) | Frontalis dystoina (n = 1; 11.1%) | ||||
| Distribution of tremor phenotype | Unilateral tremor in all (n = 10; 100%) | One upper limb (n = 19; 65.5%) | All were unilateral | Unilateral upper limb (n = 6; 50%) | Distal predominance (n = 4; 44.4%) |
| Upper and lower limb (n = 2; 20%) | Upper and lower limb (n = 6; 20.7%) | Single patient (n = 1; 5.9%) had involvement of ipsilateral lower limb (dystonia) | Unilateral upper and lower limb (n = 4; 33.3%) | Proximal predominance (n = 2; 22.2%) | |
| Distal predominant (n = 1; 10%) | Both upper limbs (n = 4; 13.7%) | Bilateral upper limb tremor (n = 1; 8.3%) | Lower limb involvement (n = 2; 22.2%) | ||
| Proximal predominant (n = 3; 30%) | Tremor involved proximal and distal segment of limbs in n = 16 (55.2%), with distal involvement in n = 3 (10.3%) | Myorhythmic rest tremor (n = 12; 70.6%) | Head tremor (n = 4; 33.3%; “nono” type in one, titubatory in one, non-specified in two) | Bilateral involvement (n = 2; 22.2%) | |
| Proximal and distal (n = 7; 70%) | Distal more than proximal involvement in n = 1 (5.9%), with all others having proximal predominance | Distal predominance (n = 9; 75%) | Voice tremor (n = 1; 11.1%) | ||
| Proximal predominance (n = 2; 16.7%) | |||||
| Proximal and distal equal distribution (n = 1; 8.3%) | |||||
| Neuroimaging results | MRI was done in n = 9 (90%) and CT in n = 1 (10%) patient(s) | MRI was performed in n = 28 (96.5%) patients while CT scan was done in n = 1 (3.4%) patient | Abnormal brain imaging (n = 16; 94.1%) | All had abnormal neuroimaging | n = 9 (100%) patients had abnormal neuroimaging |
| SPECT was performed for 6 (n = 6; 60%) patients. Did not reveal any asymmetry | Midbrain (n = 5; 29.4%) | Midbrain (n = 4; 33.3%) | Thalamus (n = 9; 100%) | ||
| Single region was involved in n = 6 (60%), more than one region n = 3 (30%) and no visible abnormality in n = 1 (10%) | Neuroimaging was abnormal in n = 28 (96.5%) | Thalamus (n = 5; 29.4%) | Thalamus (n = 4; 33.3%) | Basal ganglia (n =1; 10%) | |
| Midbrain (n = 17; 58.6%), thalamus (n = 16; 55.2%), cerebellum (n = 8; 27.6%), pons (n = 6; 20.7%), medulla (n = 1; 3.4%) | Cerebellum and/or peduncles (n = 2; 11.7%) | Cerebellum (n = 3; 25%) | Occipital lobe (n = 1;10%) | ||
| Thalamus (n = 5; 50%), midbrain (n = 3; 30%) and pons (n = 1; 10%) | Lobar (n = 3; 25%, 2 in occipital and 1 in temporal lobe) | Capsule-thalamic (n = 1; 10%) | |||
| Basal ganglia (n = 1; 8.3%) | |||||
| Cerebello-pontine angle (n = 1; 8.3%) | |||||
| Response to levodopa | n = 2 (out of 6; 33.3%) patients treated with levodopa experienced improvement at doses of 150 mg/day (additional treatment with piribedil 150 mg/day) and 400 mg/day (additional treatment with propranolol 40 mg/day) respectively | Levodopa was tried in n = 24 patients (82.8%) with improvement noted in n = 13 (out of 24; 54.2%) | Improvement noted in n = 5 (out of n = 16) patients who took levodopa (31.2%) | n = 12 (100%) patients were treated with levodopa | n = 9 (100%) patients were administered multi-drug therapy, with levodopa (up to 450 mg/day) added first and other drugs added sequentially in various combination and doses (trihexyphenidyl, clonazepam, topiramate and levetiracetam) |
| n = 9 (75%) patients showed improvement | |||||
| Two patients were additionally treated with topiramate (100 mg/day) and pramipexole (1.5 mg/day) respectively | n = 1 patient developed levodopa induced dyskinesia of upper limb which was treated with amantadine (100 mg, three times/day) | Improvement was noted in n = 5 (55.5%) patients |
| Responders (n = 5) | Non-responders (n = 4) | p value | |
|---|---|---|---|
| Mean TETRAS (PS) before therapy | 19.0 ± 3.5 | 19.3 ± 3.8 | 0.920 |
| Mean TETRAS (PS) after therapy | 9.6 ± 1.1 | 16.8 ± 4.4 | 0.014* |
| Mean TETRAS (PS) percentage reduction | 48.8 ± 6.3 | 13.8 ± 8.4 | 0.014* |
| Mean TETRAS (ADLS) before therapy | 31.2 ± 10.2 | 33.8 ± 12.5 | 0.623 |
| Mean TETRAS (ADLS) after therapy | 17.8 ± 6.9 | 30.8 ± 13.4 | 0.142 |
| Mean TETRAS (ADLS) percentage reduction | 44.4 ± 6.8 | 11.4 ± 11.2 | 0.014* |
| Levodopa dose (mean), mg/day | 240 ± 54.7 | 400 ± 40.8 | 0.012* |
| Latency (in weeks) | 7.4 ± 4.3 | 6.3 ± 4.0 | 0.705 |
| Study (n = number of participants) | Aetiology and anatomy of lesion | Functional imaging and ligand used | Results of functional imaging | Response to therapy |
|---|---|---|---|---|
| Remy et al., [7] 1995 (n = 6) | Head injury (n = 2) | [18F-DOPA] PET | Marked decrease in ipsilateral striatal uptake | All patients responded to levodopa (dramatic response in n = 2, good response in n = 2, and partial response in n = 2) |
| Bullet injury (n = 1) | [76Br]PET | |||
| Haemorrhagic stroke (n = 3) | No asymmetry of D2 uptake | |||
| All lesions in midbrain | ||||
| Paviour et al., [29] 2006 (n = 1) | Midbrain cavernoma | DAT scan (ligand not specified) | Markedly decreased striatal uptake ipsilateral to lesion | No response to levodopa |
| Hertel et al., [30] 2006 (n = 1) | Midbrain (aetiology not specified) | IBZM-SPECT | Normal post and pre-synaptic uptake | Responded well to surgical therapy (VP shunt followed by DBS) |
| [123I]-beta-CIT-SPECT (DAT Scan) | ||||
| Strecker et al., [31] 2007 (n = 1) | Midbrain abscess | DAT scan (123I SPECT) | Decreased uptake on putamen ipsilateral to lesion | Good response (almost complete amelioration of rest tremor with some persistence of kinetic tremor) |
| Guedj et al., [32] 2007 (n = 1) | Head trauma | DAT scan (FP CIT SPECT) | No striatal uptake | Excellent response to DBS (target: VIM) |
| Cerebral peduncle | ||||
| Seidel et al., [4] 2009 (n = 1) | Brainstem cavernoma bleed (left midbrain and pons) | DAT scan (Beta-CIT SPECT) | No striatal uptake | Good response to dopaminergic therapy |
| Sung et al., [33] 2009 (n = 1) | Left thalamic haemorrhage | 99mTc-TRODAT-1 SPECT | Bilateral decrease in striatal uptake (more ipsilateral to lesion) | Poor response to dopaminergic therapy |
| Gajos et al., [2] 2010 (n = 10) | Ischemic stroke-4 | [123I] DAT scan (SPECT) | No asymmetry of uptake | 6 patients treated with levodopa, 2 improved |
| Hemorrhage-6 (2 TBI, 2 ICH, 1 cavernoma, 1 AVM) | ||||
| 4 thalamus | ||||
| 5 brainstem | ||||
| 1 no lesion visible | ||||
| Reese et al., [34] 2011 (n = 1) | TBI (subdural hemorrhage) | DAT scan | Marked reduction in uptake in contralateral brain hemisphere | Transient response to dopaminergic therapy |
| 123I-FP-CIT SPECT | Treated with DBS (target VIM and STN) | |||
| Good response to surgical therapy | ||||
| Juri et al., [35] 2015 (n = 1) | Midbrain cavernoma bleed | 18F-PR04.MZ PET | Marked reduction in uptake at ipsilateral striatum | Marked response to levodopa/carbidopa |
| Gajos et al., [36] 2017 (n = 3) | 2 thalamic ischemic stroke 1 TBI | [123I]-FP CIT—DaTSCAN | Normal pre and post synaptic uptake | 2 patients treated with levodopa did not improve |
| IBZM SPECT |
18F-DOPA, 18-Fluorodopa; PET, positron emission tomogram; 76Br, bromolisuride; DAT, dopamine active transporter; IBZM, 123I-iodobenzamide; SPECT, single photon emission computed tomography; 123I-FP, ioflupane; 123I-FP-CIT, 123-radiolabeled 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane; DBS, deep brain stimulation; VIM, ventral intermediate nucleus of thalamus; TBI, traumatic brain injury; ICH, intracerebral hemorrhage; AVM, arterio-veinous malformation; STN, subthalamic nucleus.
- 1. Joutsa J, Shih LC, Fox MD. Mapping holmes tremor circuit using the human brain connectome. Ann Neurol 2019;86:812–820.ArticlePubMedPMCPDF
- 2. Gajos A, Bogucki A, Schinwelski M, Sołtan W, Rudzińska M, Budrewicz S, et al. The clinical and neuroimaging studies in Holmes tremor. Acta Neurol Scand 2010;122:360–366.ArticlePubMed
- 3. Raina GB, Cersosimo MG, Folgar SS, Giugni JC, Calandra C, Paviolo JP, et al. Holmes tremor: clinical description, lesion localization, and treatment in a series of 29 cases. Neurology 2016;86:931–938.ArticlePubMedPMC
- 4. Seidel S, Kasprian G, Leutmezer F, Prayer D, Auff E. Disruption of nigrostriatal and cerebellothalamic pathways in dopamine responsive Holmes’ tremor. J Neurol Neurosurg Psychiatry 2009;80:921–923.ArticlePubMed
- 5. Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors. from the task force on tremor of the international parkinson and movement disorder society. Mov Disord 2018;33:75–87.ArticlePubMedPMCPDF
- 6. Nsengiyumva N, Barakat A, Macerollo A, Pullicino R, Bleakley A, Bonello M, et al. Thalamic versus midbrain tremor; two distinct types of Holmes’ Tremor: a review of 17 cases. J Neurol 2021;268:4152–4162.ArticlePubMedPDF
- 7. Remy P, de Recondo A, Defer G, Loc’h C, Amarenco P, Planté-Bordeneuve V, et al. Peduncular ‘rubral’ tremor and dopaminergic denervation: a PET study. Neurology 1995;45(3 Pt 1):472–477.ArticlePubMed
- 8. Artusi CA, Farooqi A, Romagnolo A, Marsili L, Balestrino R, Sokol LL, et al. Deep brain stimulation in uncommon tremor disorders: indications, targets, and programming. J Neurol 2018;265:2473–2493.ArticlePubMedPMCPDF
- 9. Holmes G. On certain tremors in organic cerebral lesions. Brain 1904;27:327–375.Article
- 10. Krystkowiak P, Martinat P, Cassim F, Pruvo JP, Leys D, Guieu JD, et al. Thalamic tremor: correlations with three-dimensional magnetic resonance imaging data and pathophysiological mechanisms. Mov Disord 2000;15:911–918.ArticlePubMed
- 11. Martins WA, Marrone LC, Fussiger H, Vedana VM, Cristovam Rdo A, Taietti MZ, et al. Holmes’ tremor as a delayed complication of thalamic stroke. J Clin Neurosci 2016;26:158–159.ArticlePubMed
- 12. Lehéricy S, Grand S, Pollak P, Poupon F, Le Bas JF, Limousin P, et al. Clinical characteristics and topography of lesions in movement disorders due to thalamic lesions. Neurology 2001;57:1055–1066.ArticlePubMed
- 13. Kakei S, Manto M, Tanaka H, Mitoma H. Pathophysiology of cerebellar tremor: the forward model-related tremor and the inferior olive oscillationrelated tremor. Front Neurol 2021;12:694653.ArticlePubMedPMC
- 14. Alarcón F, Zijlmans JC, Dueñas G, Cevallos N. Post-stroke movement disorders: report of 56 patients. J Neurol Neurosurg Psychiatry 2004;75:1568–1574.ArticlePubMedPMC
- 15. Kim JS. Delayed onset mixed involuntary movements after thalamic stroke: clinical, radiological and pathophysiological findings. Brain 2001;124(Pt 2):299–309.PubMed
- 16. Vidailhet M, Jedynak CP, Pollak P, Agid Y. Pathology of symptomatic tremors. Mov Disord 1998;13 Suppl 3:49–54.ArticlePubMed
- 17. Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord 1994;9:493–507.ArticlePubMed
- 18. Shi M, Wang A, Fang Y, Guo J, Li Z, Jin S, et al. Study on the pathogenesis of holmes tremor by multimodal 3D medical imaging: case reports of three patients. BMC Neurol 2021;21:473.ArticlePubMedPMCPDF
- 19. Milosevic L, Kalia SK, Hodaie M, Lozano AM, Popovic MR, Hutchison WD. Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain 2018;141:2142–2155.ArticlePubMedPMC
- 20. Freeman A, Ciliax B, Bakay R, Daley J, Miller RD, Keating G, et al. Nigrostriatal collaterals to thalamus degenerate in parkinsonian animal models. Ann Neurol 2001;50:321–329.ArticlePubMed
- 21. Wang KL, Wong JK, Eisinger RS, Carbunaru S, Smith C, Hu W, et al. Therapeutic advances in the treatment of holmes tremor: systematic review. Neuromodulation 2022;25:796–803.ArticlePubMedPDF
- 22. Mishra A, Pandey S. Clinical features, neuroimaging, and levodopa-responsiveness in holmes’ tremor: a video-based case-series with a review of the literature. Mov Disord Clin Pract 2022;9:805–815.ArticlePubMedPMCPDF
- 23. Takahashi T, Kanamori H, Shigehara R, Takahashi SN, Tamura M, Takasu T, et al. Pure hemi-chorea resulting from an acute phase of contralateral thalamic lacunar infarction: a case report. Case Rep Neurol 2012;4:194–201.ArticlePubMedPMC
- 24. Frackowiak RS, Weiller C, Chollet F. The functional anatomy of recovery from brain injury. Ciba Found Symp 1991;163:235–244.ArticlePubMed
- 25. Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord 2003;18:231–240.ArticlePubMedPDF
- 26. Kaji R. Basal ganglia as a sensory gating devise for motor control. J Med Invest 2001;48:142–146.PubMed
- 27. Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol 2014;13:100–112.ArticlePubMedPMC
- 28. Koziol LF, Budding DE, Chidekel D. From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum 2012;11:505–525.ArticlePubMedPDF
- 29. Paviour DC, Jäger HR, Wilkinson L, Jahanshahi M, Lees AJ. Holmes tremor: application of modern neuroimaging techniques. Mov Disord 2006;21:2260–2262.ArticlePubMed
- 30. Hertel F, Züchner M, Decker C, Erken E, Libri S, Schmitt M, et al. Unilateral Holmes tremor, clearly responsive to cerebrospinal fluid release, in a patient with an ischemic midbrain lesion and associated chronic hydrocephalic ventricle enlargement. Case report. J Neurosurg 2006;104:448–451.PubMed
- 31. Strecker K, Schneider JP, Sabri O, Wegner F, Then Bergh F, Schwarz J, et al. Responsiveness to a dopamine agent in Holmes tremor--case report. Eur J Neurol 2007;14:e9–e10.Article
- 32. Guedj E, Witjas T, Azulay JP, de Laforte C, Peragut JC, Mundler O. Neuroimaging findings in a case of Holmes tremor. Clin Nucl Med 2007;32:139–140.ArticlePubMed
- 33. Sung YF, Hsu YD, Huang WS. (99m)Tc-TRODAT-1 SPECT study in evaluation of Holmes tremor after thalamic hemorrhage. Ann Nucl Med 2009;23:605–608.ArticlePubMedPDF
- 34. Reese R, Herzog J, Falk D, Lützen U, Pinsker MO, Mehdorn HM, et al. Successful deep brain stimulation in a case of posttraumatic tremor and hemiparkinsonism. Mov Disord 2011;26:1954–1955.ArticlePubMed
- 35. Juri C, Chana P, Kramer V, Pruzzo R, Amaral H, Riss PJ, et al. Imaging nigrostriatal dopaminergic deficit in holmes tremor with 18F-PR04.MZPET/CT. Clin Nucl Med 2015;40:740–741.ArticlePubMed
- 36. Gajos A, Budrewicz S, Koszewicz M, Bieńkiewicz M, Dąbrowski J, Kuśmierek J, et al. Is nigrostriatal dopaminergic deficit necessary for Holmes tremor to develop? The DaTSCAN and IBZM SPECT study. J Neural Transm (Vienna) 2017;124:1389–1393.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite