Articles
- Page Path
- HOME > J Mov Disord > Volume 16(3); 2023 > Article
-
Letter to the editor
Dystonic Opisthotonus in Kufor-Rakeb Syndrome: Expanding the Phenotypic and Genotypic Spectrum -
Sandeep Gurram1*
 , Vikram V Holla1*
, Vikram V Holla1* , Riyanka Kumari2,3
, Riyanka Kumari2,3 , Debjyoti Dhar1
, Debjyoti Dhar1 , Nitish Kamble1
, Nitish Kamble1 , Ravi Yadav1
, Ravi Yadav1 , Babylakshmi Muthusamy2,3
, Babylakshmi Muthusamy2,3
 , Pramod Kumar Pal1
, Pramod Kumar Pal1

-
Journal of Movement Disorders 2023;16(3):343-346.
DOI: https://doi.org/10.14802/jmd.23098
Published online: July 25, 2023
1Department of Neurology, National Institute of Mental Health and Neurosciences, Bengaluru, India
2Institute of Bioinformatics, Bengaluru, India
3Manipal Academy of Higher Education, Manipal, India
- Corresponding author: Pramod Kumar Pal, MD, DNB, DM, FRCP Department of Neurology, National Institute of Mental Health and Neurosciences, Hosur Road, Bengaluru 560029, India / Tel: +91-80-26995147 / Fax: +91-80-26564830 / E-mail: palpramod@hotmail.com
- Corresponding author: Babylakshmi Muthusamy, PhD Institute of Bioinformatics, 7th floor, Discovery Building, International Technology Park, Bengaluru 560066, India / Tel: +91-63-60297608 / E-mail: babylakshmi@ibioinformatics.org
- *These authors contributed equally to this work.
Copyright © 2023 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Dear Editor,
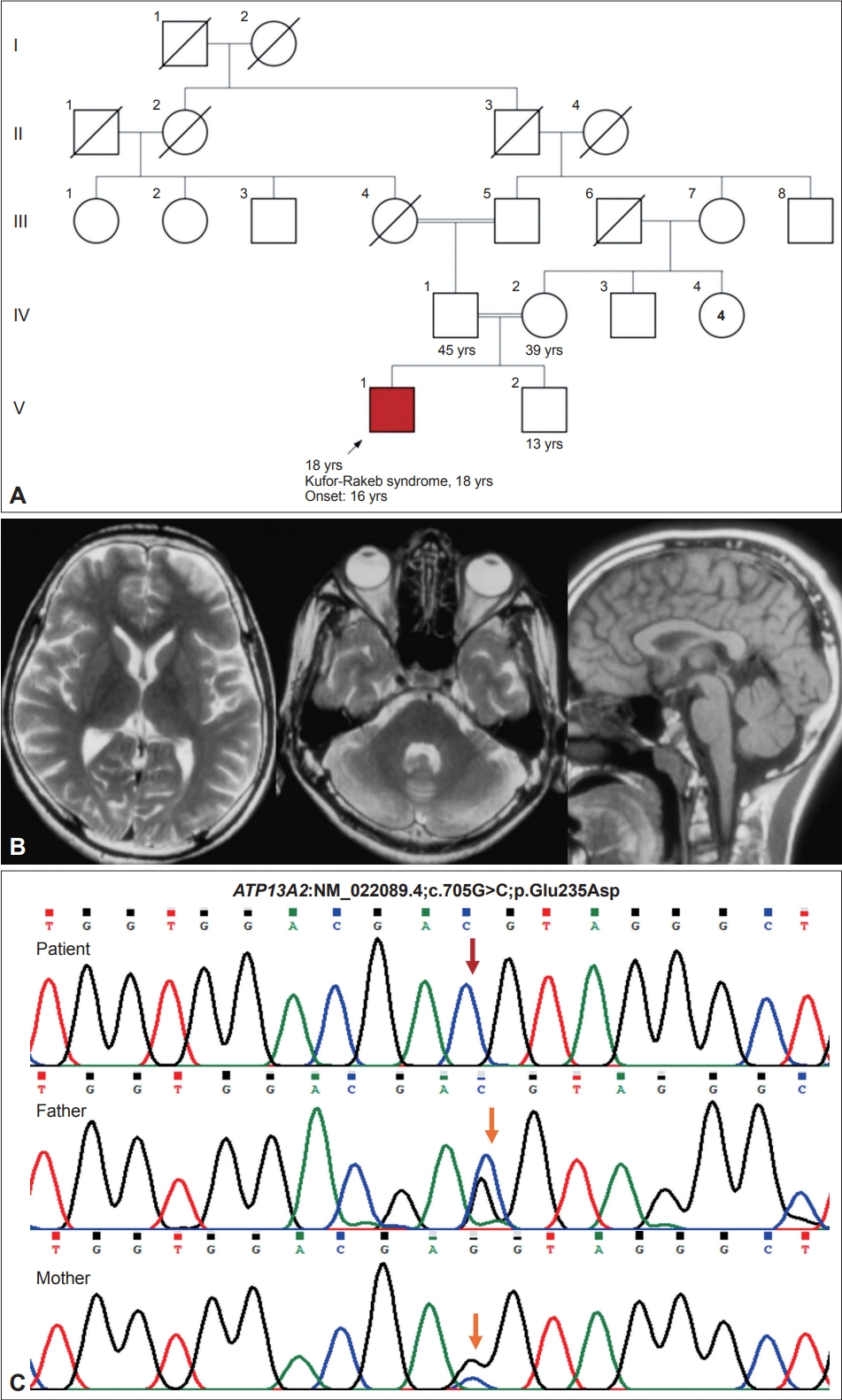
- Kufor-Rakeb syndrome is a rare autosomal recessive disease, first described in 1994 [1] in the Middle-Eastern country of Jordan. In 2006, biallelic mutations in the ATP13A2 gene were determined to be the underlying genetic etiology [2]. More than 50 cases have been reported, including four cases from India [3,4]. This syndrome is clinically characterized by juvenile-onset parkinsonism, supranuclear upgaze palsy, cognitive decline, pyramidal signs, visual hallucinations, oculogyric crisis, facial-faucial-finger mini myoclonus and dystonia in various combinations [5-7]. In addition, biallelic loss-of-function mutations in the ATP13A2 gene can result in neuronal ceroid lipofuscinosis and complicated hereditary spastic paraplegia type 78 (SPG78) [8,9]. Here, we expand the phenotypic and genotypic spectrum of Kufor-Rakeb syndrome by reporting dystonic opisthotonus in a patient with juvenile-onset parkinsonism and oculogyric crisis and a novel homozygous variant (NM_022089.4;c.705G>C) in the ATP13A2 gene.
- An 18-year-old man, born of a 3rd-degree consanguineous marriage (Figure 1), presented with episodes of uprolling of the eyeballs with retained awareness suggestive of oculogyric crisis, slurred speech, and drooling for 2 years and abnormal backward posturing of the trunk and neck while walking for 18 months. These symptoms occurred more frequently in the evenings and were reduced after a short nap. On examination, the patient had normal cognition, hypophonic speech, a reduced blink rate, mild upgaze impairment, and normal saccades and pursuits. He had right-side predominant asymmetrical parkinsonism with rigidity and bradykinesia but no tremor or postural instability. On walking, the patient had dystonic opisthotonus, which was less apparent when standing (Supplementary Video 1 in the online-only Data Supplement, Segment 1). In addition, the patient had hyperreflexia in the lower limbs with normal power and plantar response. The rest of the neurological and systemic examination results were normal.
- Routine blood investigation results, including hemogram, renal and liver function tests, serum electrolytes, copper and ceruloplasmin and magnetic resonance imaging (MRI) of the brain, were normal (Figure 1). Whole-exome sequencing revealed a novel homozygous missense variant in the ATP13A2 gene (NM_022089.4;c.705G>C;p.Glu235Asp). No other significant variant was found that could explain the clinical findings. The asymptomatic parents were found to be heterozygous carriers (Figure 1). The c.705G>C variant is novel and not reported in population databases. Computational prediction tools predicted that the p.Glu235Asp variant is likely to have functional consequences. According to Sorting Intolerant From Tolerant (SIFT), the variant was predicted to be deleterious (score = 0); PolyPhen-2 predicted it to be damaging (score = 0.983), and the Combined Annotation Dependent Depletion (CADD) score was 35. Following the guidelines established by the American College of Medical Genetics and Genomics, this variant was classified as a variant of unknown significance (PM2; PP3). However, we observed that the Glu235 residue is conserved across different species, further supporting its functional importance. In addition, this residue resides within the N-terminal conserved cation transporter/ATPase domain (188-256). Upon manual inspection of the variant in the Integrative Genomics Viewer (Broad institute, Cambridge, MA, USA and the Regents of the University of California, Oakland, CA, USA), it was observed that the variant is located at the last nucleotide of exon 8 of the ATP13A2 gene, which may potentially affect the splice donor site. To assess the impact of the variant on splicing, various prediction algorithms were employed. MutationTaster (Berlin Institute of Health at Charité, Berlin, Germany) predicted this variant to be disease-causing (score: 0.99) and suggested that it may disrupt the splice donor site, thereby affecting normal splicing. Furthermore, SpliceAI (Illumina, San Diego, CA, USA) and dbscSNV predicted this variant to be splice-altering and deleterious, with scores of 0.82 and 1, respectively. The human GRCh38.p14 Primary Assembly reports three experimental and 40 predicted transcripts for the ATP13A2 gene derived from alternate splicing. Notably, exons 7, 8, and 9 remain unchanged across all these transcripts. Therefore, the predicted splice defect is expected to be consistent across all transcripts. Notably, while multiple splice effect prediction tools suggested that the variant may cause a splice defect, experimental validation using patient samples is required to accurately determine the splice site variant’s consequences. Unfortunately, due to the unavailability of patient samples at present, this analysis could not be performed. Based on the overall clinical and genetic findings, a diagnosis of Kufor-Rakeb syndrome was made and managed accordingly. The patient significantly improved with levodopa/carbidopa (300/75 mg/day) (Supplementary Video 1 in the online-only Data Supplement, Segment 2). At the one-year follow-up, he developed levodopa-induced dyskinesias involving the face, head, and upper extremities. Levodopa/carbidopa was changed to pramipexole (1.5 mg/day) with a reasonable reduction in dyskinesias.
- Dystonia of varying severity is observed in most patients with Kufor-Rakeb syndrome [3,10]. Oculogyric crisis has been reported in many cases of Kufor-Rakeb syndrome and is associated with other classical features [1,6,11]. There have been many reports of Kufor-Rakeb syndrome associated with dystonia involving the neck and extremities other than classical oculogyric dystonic spasms [4,12]. However, opisthotonus as one of the major dystonic manifestations has not been reported to date. Abnormal backward posturing of the trunk, also termed dystonic opisthotonus, carries unique importance in the assessment of the clinical phenomenology of dystonia. The presence of dystonic opisthotonus usually suggests specific etiologies, such as drug-induced tardive dystonia; neurodegeneration with brain iron accumulation; Wilson’s disease; dopa-responsive dystonia (guanosine triphosphate cyclohydrolase-1 deficiency, tyrosine hydroxylase deficiency, aromatic L-amino acid decarboxylase deficiency, and sepiapterin reductase deficiency); and neurometabolic disorders, such as Lesch-Nyhan syndrome, glutaric aciduria and maple syrup urine disease [13]. Other causes include meningitis or encephalitis, Niemann pick type C, tetanus, strychnine poisoning, psychogenic causes and primary extensor truncal dystonia [13,14]. The association of oculogyric crisis with dystonic opisthotonus narrows down the differential diagnosis to dopa-responsive dystonia or drug-induced dystonia (especially neuroleptics). This case highlights dystonic opisthotonus associated with oculogyric crisis as the predominant manifestation of Kufor-Rakeb syndrome and indicates that it should be considered in the differential diagnosis of dystonic opisthotonus. It also emphasizes the importance of genetic testing in identifying the ever-growing newer phenotypes of various genetic diseases, especially those with variable clinical presentations and where acquired cases have been reasonably ruled out or are less likely.
- Brain MRI in Kufor-Rakeb syndrome usually shows diffuse cerebral and cerebellar atrophy with occasional evidence of iron accumulation in the basal ganglia seen as T2 hypointensities in gradient echo sequences or susceptibility-weighted imaging [5]. Our patient showed normal brain MRI, probably because the imaging was acquired early in the course of the disease. Treatment includes anticholinergics, levodopa/carbidopa, and dopamine agonists (if there is no significant cognitive decline). One patient from India had a good short-term outcome after bilateral globus pallidi deep brain stimulation, but the long-term follow-up is yet unknown [4]. The disease usually progresses rapidly during the initial part of the illness, followed by a slowly progressive course, but significant variation occurs in patients with various mutations. Usually, patients with missense mutations are reported to have slower progression, and patients with frameshift mutations vary from slow to rapid progression [5].
- In conclusion, Kufor-Rakeb syndrome can present with dystonia parkinsonism with oculogyric crisis mimicking dopa-responsive dystonia. The presence of dystonic opisthotonus is a novel clinical finding that can be observed in Kufor-Rakeb syndrome.
Supplementary Material
Video 1.
-
Ethics Statement
The authors confirm that approval from an institutional review board of National Institute of Mental Health and Neurosciences was obtained for this work (No. NIMHANS/24th IEC [BS & NS DIV.]/2020 dated 25-06-2020). We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. We also confirm that the patient has given written informed consent for video recording and publication.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
This study was partially funded by Parkinson’s Disease and Movement Disorders Research Fund and Indian Council of Medical Research (No. 54/3/2020-HUM/BMS).
-
Author contributions
Conceptualization: Sandeep Gurram, Vikram V Holla, Pramod Kumar Pal. Data curation: Sandeep Gurram, Vikram V Holla, Riyanka Kumari, Debjyoti Dhar, Babylakshmi Muthusamy, Pramod Kumar Pal. Formal analysis: Sandeep Gurram, Vikram V Holla, Riyanka Kumari, Debjyoti Dhar, Babylakshmi Muthusamy, Pramod Kumar Pal. Funding acquisition: Vikram V Holla, Nitish Kamble, Ravi Yadav, Babylakshmi Muthusamy, Pramod Kumar Pal. Investigation: Sandeep Gurram, Vikram V Holla, Riyanka Kumari, Babylakshmi Muthusamy, Pramod Kumar Pal. Methodology: Vikram V Holla, Nitish Kamble, Ravi Yadav, Babylakshmi Muthusamy, Pramod Kumar Pal. Resources: Sandeep Gurram, Vikram V Holla, Babylakshmi Muthusamy, Pramod Kumar Pal. Supervision: Nitish Kamble, Ravi Yadav, Babylakshmi Muthusamy, Pramod Kumar Pal. Validation: Vikram V Holla, Babylakshmi Muthusamy, Pramod Kumar Pal. Visualization: Vikram V Holla, Babylakshmi Muthusamy, Pramod Kumar Pal. Writing—original draft: Sandeep Gurram, Vikram V Holla. Writing—review & editing: Riyanka Kumari, Debjyoti Dhar, Nitish Kamble, Ravi Yadav, Babylakshmi Muthusamy, Pramod Kumar Pal.
Notes
- 1. Najim al-Din AS, Wriekat A, Mubaidin A, Dasouki M, Hiari M. Pallidopyramidal degeneration, supranuclear upgaze paresis and dementia: Kufor-Rakeb syndrome. Acta Neurol Scand 1994;89:347–352.ArticlePubMed
- 2. Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 2006;38:1184–1191.ArticlePubMedPDF
- 3. Wittke C, Petkovic S, Dobricic V, Schaake S, Respondek G, Weissbach A, et al. Genotype–phenotype relations for the atypical parkinsonism genes: MDSGene systematic review. Mov Disord 2021;36:1499–1510.ArticlePubMedPMCPDF
- 4. Kola S, Meka SSL, Syed TF, Kandadai RM, Alugolu R, Borgohain R. Kufor Rakeb syndrome with novel mutation and the role of deep brain stimulation. Mov Disord Clin Pract 2022;9:1003–1007.ArticlePubMedPMCPDF
- 5. Park JS, Blair NF, Sue CM. The role of ATP13A2 in Parkinson’s disease: clinical phenotypes and molecular mechanisms. Mov Disord 2015;30:770–779.ArticlePubMedPDF
- 6. Williams DR, Hadeed A, al-Din AS, Wreikat AL, Lees AJ. Kufor Rakeb disease: autosomal recessive, levodopa-responsive parkinsonism with pyramidal degeneration, supranuclear gaze palsy, and dementia. Mov Disord 2005;20:1264–1271.ArticlePubMed
- 7. Behrens MI, Brüggemann N, Chana P, Venegas P, Kägi M, Parrao T, et al. Clinical spectrum of Kufor-Rakeb syndrome in the Chilean kindred with ATP13A2 mutations. Mov Disord 2010;25:1929–1937.ArticlePubMed
- 8. Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ. Mutation of the parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum Mol Genet 2012;21:2646–2650.ArticlePubMedPMC
- 9. Estrada-Cuzcano A, Martin S, Chamova T, Synofzik M, Timmann D, Holemans T, et al. Loss-of-function mutations in the ATP13A2/PARK9 gene cause complicated hereditary spastic paraplegia (SPG78). Brain 2017;140:287–305.ArticlePubMedPMC
- 10. Yang X, Xu Y. Mutations in the ATP13A2 gene and parkinsonism: a preliminary review. Biomed Res Int 2014;2014:371256.ArticlePubMedPMCPDF
- 11. Gowda VK, Srinivasan VM, Shivappa SK. Kufor-Rakeb syndrome/Parkinson disease type 9. Indian J Pediatr 2020;87:231–232.ArticlePubMedPDF
- 12. Abbas MM, Govindappa ST, Sheerin UM, Bhatia KP, Muthane UB. Exome sequencing identifies a novel homozygous missense ATP13A2 mutation. Mov Disord Clin Pract 2017;4:132–135.PubMed
- 13. Stamelou M, Lai SC, Aggarwal A, Schneider SA, Houlden H, Yeh TH, et al. Dystonic opisthotonus: a “red flag” for neurodegeneration with brain iron accumulation syndromes? Mov Disord 2013;28:1325–1329.ArticlePubMedPMCPDF
- 14. Mainka T, Kurvits L, Skorvanek M, Necpal J, Grofik M, Ganos C. Backward leaning during gait: an underrecognized sign in Niemann-Pick type C. Parkinsonism Relat Disord 2022;101:96–98.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Estimation of Ambulation and Survival in Neurodegeneration with Brain Iron Accumulation Disorders
Elahe Amini, Mohammad Rohani, Anthony E. Lang, Zahra Azad, Seyed Amir Hassan Habibi, Afagh Alavi, Gholamali Shahidi, Maziar Emamikhah, Ahmad Chitsaz
Movement Disorders Clinical Practice.2024; 11(1): 53. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite