Articles
- Page Path
- HOME > J Mov Disord > Volume 14(3); 2021 > Article
-
Original Article
The Four Square Step Test for Assessing Cognitively Demanding Dynamic Balance in Parkinson’s Disease Patients -
Jinhee Kim
 , Ilsoo Kim
, Ilsoo Kim , Ye Eun Kim
, Ye Eun Kim , Seong-Beom Koh
, Seong-Beom Koh
-
Journal of Movement Disorders 2021;14(3):208-213.
DOI: https://doi.org/10.14802/jmd.20146
Published online: May 26, 2021
Department of Neurology and Parkinson’s Disease Center, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- Corresponding author: Seong-Beom Koh, MD, PhD Department of Neurology and Parkinson’s Disease Center, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 08308, Korea / Tel: +82-2-2626-3169 / Fax: +82-8-2626-2249 / E-mail: parkison@korea.ac.kr
Copyright © 2021 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- The Four Square Step Test (FSST) is a tool that assesses dynamic balance during obstacle step-over. To date, few studies have used the FSST to measure balance in patients with Parkinson’s disease (PD). This study aimed to verify that patients with PD, even at the de novo early stage, take more time to perform the FSST and identify which factors, cognitive status or cardinal motor symptoms, are related most to FSST scores.
-
Methods
- Thirty-five newly diagnosed drug-naïve patients with PD and 17 controls completed the FSST. The Unified Parkinson’s Disease Rating Scale (UPDRS), Hoehn and Yahr (H&Y) stage, spatiotemporal gait parameters, and neuropsychological test battery were also assessed in the PD group.
-
Results
- Mean FSST performance time was 8.20 ± 1.61 seconds in patients with PD, which was significantly more than the control group (7.13 ± 1.10 seconds, p = 0.018). UPDRS part III total score and H&Y stage were not significantly associated with FSST, but among the UPDRS subscores, only the postural instability/gait disturbance subscore showed a significant association. Regarding the association between FSST and cognition, the Trail Making Test-B and the Color Word Stroop Test showed strongly inverse correlations with FSST (rho = -0.598 and -0.590, respectively). With respect to gait parameters, double support time was significantly associated with FSST score (rho = 0.342, p = 0.044); however, other parameters, including velocity and step length, were not associated with the FSST.
-
Conclusion
- The FSST can be used in the clinic to assess dynamic balance with cognitive demands even in the early stages of PD.
- Participants
- Thirty-five patients with de novo PD, as defined by standard criteria [12], were recruited from the Parkinson’s Disease Center, Korea University Guro Hospital from July 2018 to February 2019. The diagnosis of PD was confirmed by movement disorder specialists in our center and was also supported by dopamine transporter positron emission tomography (18F-FP-CIT PET).
- We only included drug-naïve de novo patients in the early disease stage who were below Hoehn and Yahr (H&Y) stage 3 (could walk without any devices or assistants) and had never been treated with dopaminergic drugs. Patients were excluded if they had dementia, definite autonomic dysfunction, downward saccadic slowness (including ”Round the houses sign”) or other red flag signs suggesting atypical parkinsonism. Patients who had a history of previous falls or performed poorly on the pull test (2 or greater score on the UPDRS part III-30) were excluded because of safety risks and the possibility they had atypical parkinsonism. Patients were also excluded if they had a history of stroke, head trauma, or other neurological and musculoskeletal diseases that could likely contribute to gait disturbances.
- The patients with PD were compared with 17 control subjects who were diagnosed with essential tremor and did not have parkinsonism. Additionally, this control grouponsisted of only those who had no gait and balance dysfunction during an examination and no comorbidity that could affect gait and balance. All information from the PD patients and controls was collected retrospectively. The Institutional Review Board at Korea University Guro Hospital approved the study, and participants signed written informed consent forms before participating.
- Procedures and measurements
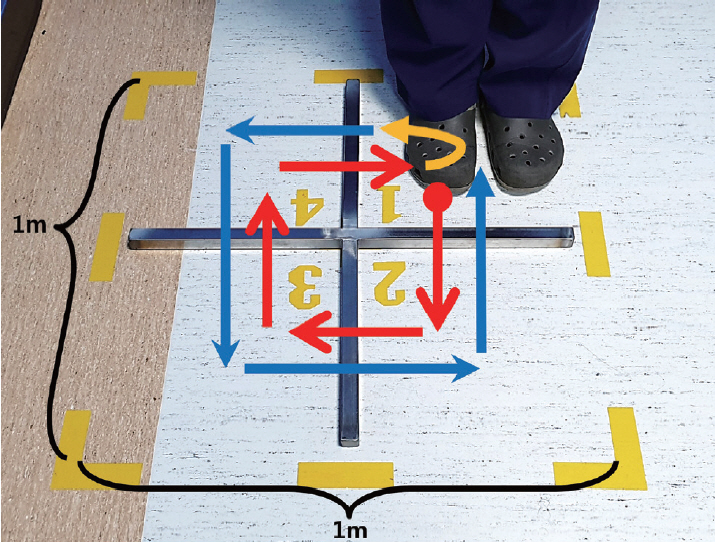
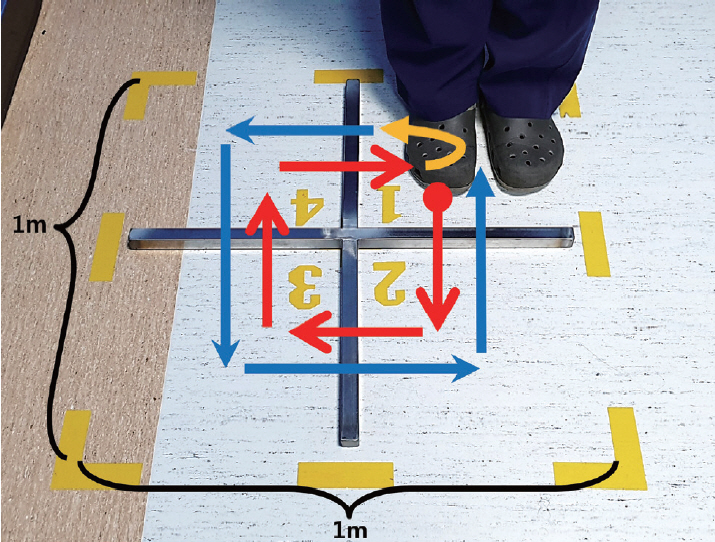
- The FSST requires participants to step over small and low obstacles (horizontal and vertical length of the obstacle 90 cm, height of the obstacle 2.5 cm, side length of the outer box border 1 m) placed in a cross configuration by generally following the initial protocol used in a previous study [6]. At the start of the test, the participants stand on the first (lower left) square with both feet close together and are instructed to take one step at a time over each obstacle in a clockwise direction as rapidly and as safely as possible, touching each square with both feet before moving to the next square: first moving forward, then to the right, backwards, then left to their original position. Then, they should immediately repeat the same steps in the counterclockwise direction (Figure 1). After one practice trial, the patient time was recorded for the next two trials, and the best time in seconds was used as the FSST score. The assessor was blinded to the participant’s group.
- UPDRS part III and H&Y stage were administered and scored by one trained rater and confirmed by three movement disorder specialists via recorded video review. The bradykinesia subscore of the UPDRS-III was calculated by summing the scores for items 15–22 and 27, and the PIGD subscore was obtained by summing items 23–26. We also assessed spatiotemporal gait parameters (i.e., gait velocity, cadence, step length, base, percentage of double and single support, and percentage of swing and stance phase during walking at a comfortable speed) using the computerized GaitRite System (CIR Systems, Inc., Franklin, NJ, USA). The Korean version of the Montreal Cognitive Assessment (K-MoCA) was administered to all patients with PD, and we also used the percentile score adjusted by age, sex and education age for each cognition test in the Seoul Neuropsychological Screening Battery (SNSB), including the Digit-Span Test (DST), the Korean version of the Boston Naming Test (K-BNT), the Rey Complex Figure Test (RCFT), the Seoul Verbal Learning Test (SVLT), the Digit Symbol Coding (DSC), the Controlled Oral Word Association Test (COWAT), the TMT-B and the Color Word Stroop Test (CWST), if it was administered.
- Statistical analysis
- Descriptive statistics were calculated for age, sex, disease duration, and education. Differences in FSST performance time between patients with PD and control groups were examined using t-tests (for continuous variables) and chi-squared or Fisher’s exact tests (for nominal variables). The FSST performance times were not normally distributed; therefore, the association between the FSST and concurrent motor and cognitive measures and spatiotemporal gait parameters were determined with Spearman’s correlation coefficient. All analyses were performed using SPSS software (IBM SPSS Statistics for Windows, version 20.0; IBM Corp., Armonk, NY, USA). All reported p values were 2-tailed. The level of significance was set at p < 0.05.
MATERIALS & METHODS
- Thirty-five patients with PD and seventeen controls participated in this study. The clinical characteristics of all participants are reported in Table 1. No falls or injuries occurred during the testing. There were no significant differences in age or sex ratio between the PD and control groups. In the PD group, the mean disease duration, which was calculated using the year of symptom onset, was 14.14 months, and the mean UPDRS part III total score was 23.8. The mean FSST performance time was 8.20 ± 1.61 seconds in the PD group, which was significantly different from that in the control group (7.13 ± 1.10 seconds, p = 0.018). The FSST performance time was significantly correlated with age (rho = 0.531, p = 0.001) and disease duration (rho = 0.356, p = 0.036) only in the PD group (Table 2).
- In terms of motor symptoms and disease severity, the UPDRS part III total score and H&Y stage were not significantly associated with the FSST performance time. However, among the UPDRS subscores, the PIGD subscore showed a significant FSST performance association, but the bradykinesia subscore did not (Table 2). Next, we intended to elucidate the association between the FSST and cognition, but the K-MoCA score was not associated with FSST performance. After analyzing the results of the SNSB of 18 PD patients whose data for the full cognition test battery were available (Supplementary Table 1 in the online-only Data Supplement), we found there was no significant correlation between the FSST and all cognitive domains, including attention, language, memory, visuospatial, and frontal/executive function. However, an analysis of each individual test showed that TMT-B and CWST were strongly and significantly inversely correlated with FSST performance (worse TMT-B or CWST scores were associated with longer FSST performance times) (Table 3). Because in these 18 patients, disease duration was not significantly correlated with the FSST (rho = 0.258, p = 0.302) and each domain and test value was the percentile score, which already took into account the participant’s age and education level, there was no need to adjust for age and disease duration in this statistical analysis.
- Correlations between the FSST time and the spatiotemporal gait parameters are presented in Table 4. Only the percentage of double stance was significantly correlated with the FSST time, whereas velocity, cadence, step length, and the coefficient of variation of step length and base were not statistically significantly correlated with FSST time.
RESULTS
- To our knowledge, this is the first study to examine whether patients with PD show differences in FSST performance even when they are drug-naïve and in the early disease stage. According to our results, patients with PD, even in the early disease stage, showed slower FSST performance times than the control group. The mean performance time measured in this study was slightly faster than that in previous studies (8.20 sec in ours vs. 9.52 sec in [10] and 12.9 sec in [11]), which may be due to the early disease stage and short disease duration in our participants. The FSST has been used as a balance test to anticipate falling tendency in healthy older adults, and among those with vestibular disorders, musculoskeletal conditions such as joint replacement and osteoporosis, and CNS disorders such as stroke, multiple sclerosis and Huntington’s disease [13].
- There are two studies so far that have used the FSST in patients with PD. In these studies, the reliability and validity of the FSST were established, but it was not as good as other tests (e.g., the Mini-BESTest) at distinguishing between fallers and nonfallers [10]. The FSST performance time was correlated with disease severity, as measured by both the UPDRS and H&Y stage [11]. Unlike our study, these previous studies were conducted in patients with mild to moderate PD who had already been diagnosed with PD and were taking dopaminergic drugs (mean disease duration 6.25 years, mean UPDRS part-III score 28.6), including some fallers or freezers. Our results obtained in newly diagnosed patients with early-stage PD showed that FSST performance was not associated with the severity of motor symptoms such as bradykinesia and was also not associated with spatiotemporal gait parameters (i.e., velocity, cadence, and step length in gait analysis). Instead, frontal/executive function (TMT-B and CWST) was strongly correlated with the FSST performance. Considering our results that the UPDRS PIGD subscore and double support time in gait analysis were correlated with FSST performance, the FSST acts primarily as a balance test, as it has been used so far. Therefore, we can suggest that the FSST functions as a balance test that incorporates some cognitive functionality.
- There are two distinct types of balance, simple static and dynamic balance. The latter has more meaning of the ability of a person to balance while in motion or while switching between positions. Past studies have described balance impairment and falling as the two main motor problems that are observed in the advanced stages of PD, and they are accompanied by the progression of other cardinal motor symptoms. If balance impairment instruments such as the pull test and tandem gait test are abnormal in the early disease stage, this is thought to suggest atypical parkinsonism [14]. However, falling in PD is not only a simple static balance problem when standing or walking but also a dynamic balance problem when turning, obstacle-crossing, and during dual-task performance. Dynamic balance is multidimensionally affected by lower extremity muscle strength, sensory integration ability, and frontal/executive functions, as well as parkinsonian motor symptoms [15,16]. Above all, previous articles reported that executive functions, particularly those involving set shifting and inhibition, were associated with worse balance and gait performance in patients with PD [4]. Based on our studies, we proposed that even though it is not overtly obvious in patients’ daily living, impairment of dynamic balance also exists in the early stage of PD, and this dynamic imbalance in patients with early-stage PD is mainly related to frontal lobe executive dysfunction, among other factors that affect dynamic balance.
- Numerous rating scales and clinical tests that have been demonstrated to be valid and reliable as dynamic balance tests for PD are already used worldwide. Among them, several, such as the Tinetti Balance Scale, Berg Balance Test, and the mini-BESTest, are recommended or suggested by the Movement Disorder Society [17]. However, these tests cannot evaluate the cognitive components of dynamic balance that require executive function. Furthermore, while the FSST score is analyzed as a continuous variable that measures performance time in seconds, the results from the other scales or tests mentioned above show strong ceiling or floor effects [17]. In this regard, the FSST can be a useful assessment that compensates for the limitations of traditional instruments and adds the ability to evaluate dynamic balance in patients with PD.
- It is an interesting and instructive finding that FSST performance was not significantly associated with other tests of frontal/executive function (i.e., MoCA, DSC, and the COWAT) but was strongly correlated with the TMT-B and CWST. Unlike the former, the latter is more similar to the FSST process in that the latter is more visuocognitively demanding. In addition to simple visual abilities such as visual acuity, visuocognition, which requires an interaction between visual and cognitive function, is also important for gait stability and adaptability [18]. Vision and cognition have been found to be separately related to gait impairment, but the interactive effect of both has not been investigated. Knowledge of visuocognitive processes during gait is essential and important to fully understand the mechanisms underlying gait and balance impairment in patients with PD. Therefore, measurement instruments that can also reveal visuocognitive effects on gait and balance are indispensable. For this reason, a few investigators have reported using their own instruments or assessments that can explain more complex walking adaptability in the context of visuocognitive processing [19], but they are still not very feasible and have not been standardized and validated. We suggest that the FSST could be a good candidate instrument, as it is an easily administered, comprehensive gait and balance scale that assesses all relevant constructs, including executive functions and visuocognitive interactions.
- This study has several limitations. First, some information for the control group, such as education, BMI, cognitive tests, and gait parameters, was not available. This makes it challenging to determine whether worse FSST performance in the PD group than in the control group was due to worse cognitive function. Next, our sample size was somewhat small, and fully available data were limited. However, since we introduced the FSST as an easy and feasible test in this study, we planned a future study with a larger sample size that will be conducted soon. Last and the most important limitation is that we used essential tremor patients as a control group. As already mentioned in many studies, essential tremor has many features associated with gait, balance and cognition. Although we only included people who showed only an action tremor without any gait or balance problems in the examination, we admit that this could be an inevitable limitation of this research.
DISCUSSION
- In this study, we found that FSST performance was impaired even in de novo, early-stage patients with PD, and this was associated with executive function. The FSST is a feasible measurement for balance in the clinical field (quick to administer, objectively scored in seconds, requires little space and cost). Not only is it suitable for assessing dynamic balance, but it is also unique in that it requires executive function and visuocognitive processing. Therefore, administration of the FSST helps assess motor-cognitive interactions in PD and it is especially useful for investigating associations between dynamic balance and executive function.
Conclusion
Supplementary Materials
Supplementary Table 1.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Author Contributions
Conceptualization: Jinhee Kim, Seong-Beom Koh. Data curation: Jinhee Kim, Ilsoo Kim, Ye Eun Kim. Formal analysis: Jinhee Kim. Investigation: Jinhee Kim, Ilsoo Kim, Ye Eun Kim. Methodology: Jinhee Kim, Ilsoo Kim, Seong-Beom Koh. Project administration: Seong-Beom Koh. Resources: all authors. Software: Jinhee Kim. Supervision: Seong-Beom Koh. Validation: Ilsoo Kim, Ye Eun Kim. Visualization: Jinhee Kim. Writing—original draft: Jinhee Kim. Writing—review & editing: all authors.
Notes
- This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
| PD (n = 35) | Control (n = 17) | p-value | |
|---|---|---|---|
| Age | 63.63 (9.67) | 64.41 (9.76) | 0.786 |
| Sex (M/F) | 20/15 | 7/10 | 0.378 |
| Disease duration (months) | 14.14 (17.23) | ||
| Education (years) | 10.17 (4.50) | ||
| BMI* | 24.08 (2.57) | ||
| Height (cm) | 163.57 (9.32) | ||
| K-MoCA (n=34) | 25.59 (3.47) | ||
| H&Y stage | 2.09 (0.23) | ||
| UPDRS part III total score | 23.79 (9.67) | ||
| UPDRS bradykinesia subscore | 12.41 (4.59) | ||
| UPDRS PIGD subscore | 1.79 (1.10) | ||
| FSST time (secs) | 8.20 (1.61) | 7.13 (1.10) | 0.018 |
Data are shown as mean (SD) or n.
* weight (kg)/height (m2).
PD: Parkinson’s disease, SD: standard deviation, M: male, F: female, BMI: body mass index, K-MoCA: Korean-Montreal Cognitive Assessment, H&Y stage: Hoehn and Yahr stage, UPDRS: Unified Parkinson’s Disease Rating Scale, PIGD: postural instability/gait disturbance, FSST: Four Square Step Test.
| Element | rho | p-value |
|---|---|---|
| Control | ||
| Age | 0.374 | 0.139 |
| PD | ||
| Age | 0.531* | 0.001* |
| Disease duration | 0.356* | 0.036* |
| Education | -0.270 | 0.116 |
| BMI | -0.202 | 0.244 |
| Height | 0.224 | 0.197 |
| K-MoCA | -0.015 | 0.935 |
| H&Y stage | 0.251 | 0.146 |
| UPDRS part III total score | 0.307 | 0.073 |
| UPDRS bradykinesia subscore | 0.179 | 0.305 |
| UPDRS PIGD subscore | 0.445* | 0.007* |
| Domain | rho (p-value) | Tests | rho (p-value) |
|---|---|---|---|
| Attention | 0.139 (0.581) | DST | 0.051 (0.842) |
| Language | 0.447 (0.063) | K-BNT | 0.344 (0.163) |
| Visuospatial | 0.368 (0.132) | RCFT | 0.228 (0.363) |
| Memory | 0.319 (0.197) | SVLT delayed recall | 0.038 (0.189) |
| RCFT delayed recall | 0.189 (0.453) | ||
| Frontal/executive | -0.075 (0.766) | DSC | 0.108 (0.669) |
| COWAT | 0.219 (0.383) | ||
| TMT-B | -0.598 (0.009)* | ||
| CWST | -0.590 (0.010)* |
Each domain and test value are the percentile score considering participant age and education, so this was not adjusted by age in this statistical analysis.
* statistically significant.
DST: Digit-Span Test, FSST: Four Square Step Test, K-BNT: Korean version of the Boston Naming Test, RCFT: Rey Complex Figure Test, SVLT: Seoul Verbal Learning Test, DSC: Digit Symbol Coding, COWAT: Controlled Oral Word Association Test, TMT-B: Trail Making Test B, CWST: Color Word Stroop Test.
| Mean (SD) | rho | p-value | |
|---|---|---|---|
| Velocity (cm/sec) | 87.21 (16.79) | -0.303 | 0.086 |
| Cadence (steps/min) | 102.87 (10.41) | -0.234 | 0.190 |
| Swing time (% of the total GC) | |||
| Dominant side* | 34.28 (1.65) | -0.034 | 0.845 |
| Average of the right and left sides | 35.07 (1.39) | -0.159 | 0.362 |
| Average of double support time (% of the total GC) | 29.17 (2.99) | 0.342 | 0.044 |
| Step length (cm) | |||
| Differential† | 2.25 (2.03) | -0.111 | 0.539 |
| Dominant side | 49.88 (8.00) | -0.276 | 0.120 |
| Average | 51.00 (8.06) | -0.278 | 0.117 |
| CV‡ | |||
| Dominant side | 6.93 (1.98) | 0.261 | 0.142 |
| Average | 6.06 (1.67) | 0.289 | 0.103 |
| Base (cm) | |||
| Average | 8.92 (2.16) | -0.023 | 0.901 |
| Average CV‡ | 18.91 (10.36) | 0.206 | 0.249 |
* shows more severe parkinsonism.
† difference between the right and left side, which means the amount of asymmetry.
‡ coefficient of variation, which means the extent of variability in relation to the mean of the population.
FSST: Four Square Step Test, SD: standard deviation, GC: gait cycle, CV: coefficient of variation.
- 1. Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 2017;32:1264–1310.ArticlePubMedPMC
- 2. Price A, Shin JC. The impact of Parkinson’s disease on sequence learning: perceptual pattern learning and executive function. Brain Cogn 2009;69:252–261.ArticlePubMed
- 3. Kelly VE, Johnson CO, McGough EL, Shumway-Cook A, Horak FB, Chung KA, et al. Association of cognitive domains with postural instability/gait disturbance in Parkinson’s disease. Parkinsonism Relat Disord 2015;21:692–697.ArticlePubMedPMC
- 4. Xu D, Cole MH, Mengersen K, Silburn PA, Qiu F, Graepel C, et al. Executive function and postural instability in people with Parkinson’s disease. Parkinsons Dis 2014;2014:684758.ArticlePubMedPMC
- 5. Raffegeau TE, Krehbiel LM, Kang N, Thijs FJ, Altmann LJP, Cauraugh JH, et al. A meta-analysis: Parkinson’s disease and dual-task walking. Parkinsonism Relat Disord 2019;62:28–35.ArticlePubMed
- 6. Dite W, Temple VA. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch Phys Med Rehabil 2002;83:1566–1571.ArticlePubMed
- 7. Goh EY, Chua SY, Hong SJ, Ng SS. Reliability and concurrent validity of Four Square Step Test scores in subjects with chronic stroke: a pilot study. Arch Phys Med Rehabil 2013;94:1306–1311.ArticlePubMed
- 8. Kalron A, Givon U. Construct validity of the Four Square Step Test in multiple sclerosis. Arch Phys Med Rehabil 2016;97:1496–1501.ArticlePubMed
- 9. Kloos AD, Fritz NE, Kostyk SK, Young GS, Kegelmeyer DA. Clinimetric properties of the Tinetti Mobility Test, Four Square Step Test, Activitiesspecific Balance Confidence Scale, and spatiotemporal gait measures in individuals with Huntington’s disease. Gait Posture 2014;40:647–651.ArticlePubMedPMC
- 10. Duncan RP, Earhart GM. Four square step test performance in people with Parkinson disease. J Neurol Phys Ther 2013;37:2–8.ArticlePubMed
- 11. McKee KE, Hackney ME. The Four Square Step Test in individuals with Parkinson’s disease: association with executive function and comparison with older adults. NeuroRehabilitation 2014;35:279–289.ArticlePubMed
- 12. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39.ArticlePubMed
- 13. Langford Z. The Four Square Step Test. J Physiother 2015;61:162.ArticlePubMed
- 14. Nonnekes J, Aerts MB, Abdo WF, Bloem BR. Medio-lateral balance impairment differentiates between Parkinson’s disease and atypical parkinsonism. J Parkinsons Dis 2014;4:567–569.ArticlePubMed
- 15. Liao YY, Yang YR, Wu YR, Wang RY. Factors influencing obstacle crossing performance in patients with Parkinson’s disease. PLoS One 2014;9:e84245.ArticlePubMedPMC
- 16. Caetano MJD, Lord SR, Allen NE, Song J, Paul SS, Canning CG, et al. Executive functioning, muscle power and reactive balance are major contributors to gait adaptability in people with Parkinson’s disease. Front Aging Neurosci 2019;11:154.ArticlePubMedPMC
- 17. Bloem BR, Marinus J, Almeida Q, Dibble L, Nieuwboer A, Post B, et al. Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: critique and recommendations. Mov Disord 2016;31:1342–1355.ArticlePubMed
- 18. Stuart S, Lord S, Hill E, Rochester L. Gait in Parkinson’s disease: a visuocognitive challenge. Neurosci Biobehav Rev 2016;62:76–88.ArticlePubMed
- 19. Geerse DJ, Roerdink M, Marinus J, van Hilten JJ. Assessing walking adaptability in Parkinson’s disease: “The Interactive Walkway”. Front Neurol 2018;9:1096.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Impact of mobile phone usage on dynamic postural control among South Indian college students
S. Dhanusia, S. Santhana Lakshmi, Ajith Kumar, R. Prabhu, Vignesh Srinivasan, Prathap Suganthirababu, Priyadharshini Kumar, A. Kumaresan, Surya Vishnuram, Jagatheesan Alagesan, Rajkumar Krishnan Vasanthi
Work.2024; : 1. CrossRef - A Computer Vision-Based System to Help Health Professionals to Apply Tests for Fall Risk Assessment
Jesús Damián Blasco-García, Gabriel García-López, Marta Jiménez-Muñoz, Juan Antonio López-Riquelme, Jorge Juan Feliu-Batlle, Nieves Pavón-Pulido, María-Trinidad Herrero
Sensors.2024; 24(6): 2015. CrossRef - 2023 Carol B. Lewis Distinguished Lecture Address to the APTA Geriatrics Membership Combined Sections Meeting, February 23, 2023 Key Words & Challenges: Defining Our Role in Caring for Older Adults
Michelle M. Lusardi
Journal of Geriatric Physical Therapy.2023; 46(2): 93. CrossRef - The relationship between visual function and physical performance in the Study of Muscle, Mobility and Aging (SOMMA)
Atalie C. Thompson, Eileen Johnson, Michael E. Miller, Jeff D. Williamson, Anne B. Newman, Steve Cummings, Peggy Cawthon, Stephen B. Kritchevsky, Eric R. Anson
PLOS ONE.2023; 18(9): e0292079. CrossRef - Relationship between parental history of dementia, motor-cognitive and executive function performance in African American women
Allison A. Bay, Nicole Schindler, Whitney Wharton, Hayley Silverstein, Liang Ni, Todd A. Prusin, Madeleine E. Hackney
Journal of the Neurological Sciences.2022; 439: 120305. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite