Articles
- Page Path
- HOME > J Mov Disord > Volume 17(2); 2024 > Article
-
Letter to the editor
Suprahyoid Tremor as an Early Feature of Multiple System Atrophy-Parkinsonism: A Case Report -
Shakya Bhattacharjee1

 , Rohini Aggarwal2, Ronan Macdonagh3, Christopher Kobylecki1,4
, Rohini Aggarwal2, Ronan Macdonagh3, Christopher Kobylecki1,4
-
Journal of Movement Disorders 2024;17(2):242-244.
DOI: https://doi.org/10.14802/jmd.23261
Published online: March 6, 2024
1Department of Neurology, Manchester Centre for Clinical Neurosciences, Northern Care Alliance NHS Foundation Trust, Salford, UK
2Department of Otolaryngorhinology, Salford Royal Hospital NHS Foundation Trust, Salford, UK
3Department of Neurophysiology, Salford Royal Hospital NHS Foundation Trust, Salford, UK
4Division of Neuroscience and Experimental Psychology, Manchester Academic Health Science Centre, University of Manchester, Manchester, UK
- Corresponding author: Shakya Bhattacharjee, FRCP Department of Neurology, Manchester Centre for Clinical Neurosciences, Stott Lane, Salford M6 8HD, UK / Tel: +44-161-789-7373 / Fax: +44-161-206-5767 / E-mail: bubai.shakya@gmail.com
Copyright © 2024 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 403 Views
- 23 Download
- Dear Editor,
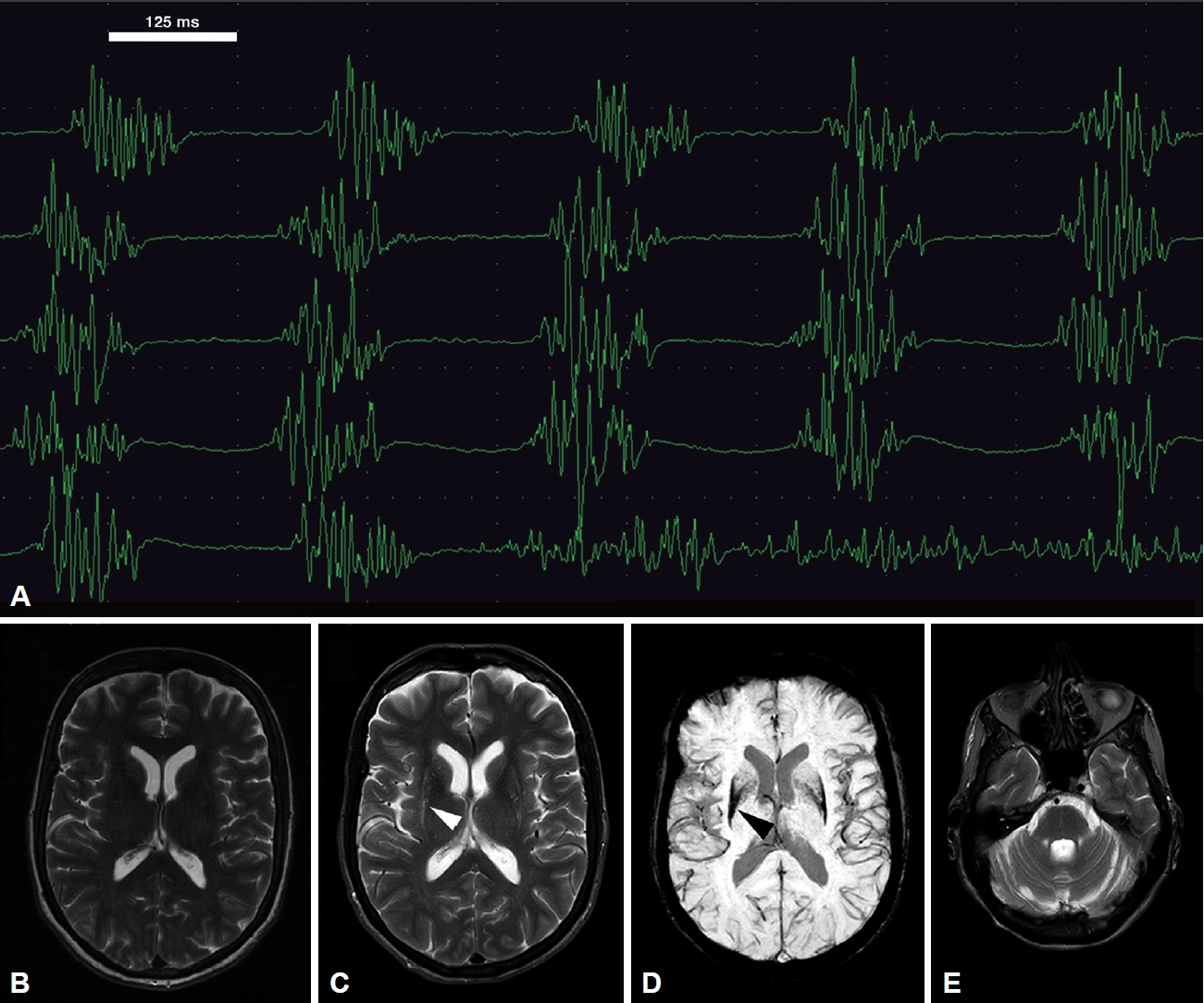
- A 61-year-old female patient presented with stiffness and slowness of the left hand with loss of dexterity for 24 months. She had no limb tremor and no problems with mobility or balance, sphincter disturbance or lightheadedness. There was no history of neuroleptic medication use. She had left upper limb rigidity and bradykinesia. A dopamine transporter scan revealed bilateral reduced tracer uptake, and she was diagnosed with idiopathic Parkinson’s disease; her magnetic resonance (MR) brain was normal (Figure 1A). Her bradykinesia and rigidity initially responded well to cobeneldopa 100/25 mg three times daily, but she reported ongoing tremulous movements at rest in her neck and throat and tongue tremor after 8–10 months of commencement with levodopa. The tremor improved with mouth opening and was somewhat less severe following a dose of levodopa. Her speech and swallowing at this point were normal. Examination during her OFF state revealed that she had resting tongue tremor, a regular rhythmic, nondistractible and nonentrainable movement suggestive of tremor in the suprahyoid muscles. She had no dystonic posturing of the tongue, jaws, or neck. The tremor occurred in both flexed and extended neck postures (Supplementary Video 1 in the online-only Data Supplement). The resting tongue and neck tremor significantly improved after 45 minutes of taking levodopa; there was subtle dystonic movement of the left side of the mouth in the ON state (Supplementary Video 2 in the online-only Data Supplement).
- The electromyogram (EMG) revealed rhythmic tremulous 5 Hz discharge of both anterior digastric muscles (Figure 1B). Regular rhythmicity, nondistractibility and a nonentrainable nature were evident during the neurophysiological study, making functional tremors unlikely. Five units of onabotulinum toxin type A were injected into each anterior digastric muscle under EMG guidance. Due to the poor response to this initial injection, a further dose of 7 units of onabotulinum toxin type A was injected into each anterior digastric muscle three months later, together with 1 unit to the left risorius muscle. She had a very good response, and a follow-up clinical review and EMG of the suprahyoid muscles six months after the last injection showed no tremor.
- Her suprahyoid tremor remained under good control following these injections, but she reported early worsening of wearing-off motor and nonmotor symptoms within three years from symptom onset, including severe anxiety, which responded poorly to adjustment of oral antiparkinsonian medications. She developed postural hypotension and worsening features of orofacial dystonia, including blepharospasm, as well as dysarthric and dysphonic speech and swallowing problems four years after suprahyoid tremor development. A repeat MR scan of the brain five years after the initial assessment revealed atrophy/putaminal slit signs and low susceptibility-weighted imaging signals in the posterolateral putamen, pontocerebellar atrophy, and patchy high T2 signals in both middle cerebellar peduncles (Figure 1C-E). The diagnosis was changed to clinically established multiple system atrophy-parkinsonism (MSA-P) according to the most recent International Parkinson and Movement Disorder Society criteria [1].
- The suprahyoid muscles consist of the anterior and posterior belies of the digastric muscles and the mylohyoid, stylohyoid and geniohyoid muscles. These muscles elevate the hyoid bone and help in swallowing. There is a paucity of literature regarding abnormal movements related to this muscle group. Baik et al. [2]reported suprahyoid and tongue tremors in three patients. Two of these reactions were triggered by levosulpiride use and were reversed upon cessation of this drug, and one was functional in nature. Fernández-Pajarín et al. [3] reported a good response of hyoid dystonia to two sessions of botulinum toxin injection. Norby et al. [4] reported a large series of hyoid dystonia in which a change in speech resonance was observed in all patients, and dysphagia was reported in 73.3% of patients, although tremor was not described. In the case described here, significant dysphagia and dysarthria occurred only four years later and were not temporally linked, suggesting that dystonia of the hyoid muscle may not have been the key mechanism involved. One case series of Meige syndrome reported movement of the suprahyoid muscles, known as “frog like” movement, in 12 patients [5]. However, this movement is different from that of the suprahyoid tremor, as our patient had rhythmic movement of the anterior belly of the digastric muscle, but frog-like movement mainly involved other suprahyoid muscles.
- To the best of our knowledge, hyoid dystonia or hyoid tremor in probable or clinically established MSA patients has never been reported in the literature. A large review on tremors in MSA patients revealed that myoclonic limb tremors are common in MSA patients, but neck or tongue tremors have not been reported [6]. Tongue tremor is more commonly observed in Parkinson’s disease [7], but to our knowledge, tremulous movements of hyoid muscles have not been reported in degenerative parkinsonian syndromes. The presence of subtle orofacial dystonia in our patient was a clue to the eventual diagnosis of MSA [1], although she did not fulfill the diagnostic criteria for MSA until later in her disease course. The mechanism of tremor in MSA is poorly understood [6], and we postulate an interaction between dopamine depletion and striatal pathology and infratentorial abnormalities such as brainstem and cerebellar involvement. Atypical tongue and suprahyoid tremor could be a presenting feature of MSA, and the presence of dystonic movements in the orofacial musculature should prompt re-evaluation of an initial diagnosis of Parkinson’s disease.
Supplementary Materials
Video 1.
Video 2.
-
Ethics Statement
The authors confirm that the an Institution Ethics Committee review waiver was obtained for this case report from the Salford Royal Hospital NHS Foundation Trust. The study was performed as per the declaration of Helsinki. A written consent was obtained from the patient for the publication of the video for this journal. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
-
Conflicts of Interest
Shakya Bhattacharjee: Travel grant from the Britannia pharmaceuticals and Ipsen Pharma. Christopher Kobylecki: Grant funding from Parkinson’s UK and Michael J Fox Foundation; speaker fees from Britannia and Bial Pharma; support to attend international meetings from Abbvie and Merz Pharma; payment for advisory boards from Britannia and Abbvie. All remaining authors have no financial conflicts of interest.
-
Funding Statement
None
-
Author Contributions
Conceptualization: Shakya Bhattacharjee, Christopher Kobylecki. Data curation: Shakya Bhattacharjee, Christopher Kobylecki. Formal analysis: Shakya Bhattacharjee, Rohini Aggarwal, Ronan Macdonagh. Investigation: all authors. Methodology: Shakya Bhattacharjee, Christopher Kobylecki. Project administration: all authors. Resources: Shakya Bhattacharjee, Christopher Kobylecki. Software: Shakya Bhattacharjee, Christopher Kobylecki. Supervision: Christopher Kobylecki. Validation: all authors. Visualization: all authors. Writing—original draft: Shakya Bhattacharjee. Writing—review & editing: all authors.
Notes
- None
Acknowledgments
- 1. Wenning GK, Stankovic I, Vignatelli L, Fanciulli A, Calandra-Buonaura G, Seppi K, et al. The Movement Disorder Society criteria for the diagnosis of multiple system atrophy. Mov Disord 2022;37:1131–1148.PubMedPMC
- 2. Baik JS, Lyoo CH, Lee JH, Lee MS. Drug-induced and psychogenic resting suprahyoid neck and tongue tremors. Mov Disord 2008;23:746–748.ArticlePubMedPDF
- 3. Fernández-Pajarín G, Martínez-Castrillo JC, Dominguez-Lorenzo JM, Vaamonde P. Invalidating hyoid dystonia: successful treatment with incobotulintoxinA. Mov Disord Clin Pract 2021;8:264–266.ArticlePubMedPMCPDF
- 4. Norby E, Orbelo D, Strand E, Duffy J, Ekbom D, Bower J, et al. Hyoid muscle dystonia: a distinct focal dystonia syndrome. Parkinsonism Relat Disord 2015;21:1210–1213.ArticlePubMed
- 5. Watson NA, Hicklin LA, Marion MH. Breathing dystonia in Meige syndrome. Clin Park Relat Disord 2021;5:100106.ArticlePubMedPMC
- 6. Kaindlstorfer C, Granata R, Wenning GK. Tremor in multiple system atrophy– a review. Tremor Other Hyperkinet Mov (N Y) 2013;3:tre-03-165-4252-1.ArticlePubMedPMC
- 7. Silverdale MA, Schneider SA, Bhatia KP, Lang AE. The spectrum of orolingual tremor--a proposed classification system. Mov Disord 2008;23:159–167.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite

