Articles
- Page Path
- HOME > J Mov Disord > Volume 17(1); 2024 > Article
-
Review Article
Fighting Against the Clock: Circadian Disruption and Parkinson’s Disease -
Yen-Chung Chen1,2
 , Wei-Sheng Wang1
, Wei-Sheng Wang1 , Simon J G Lewis3
, Simon J G Lewis3
 , Shey-Lin Wu1,4
, Shey-Lin Wu1,4

-
Journal of Movement Disorders 2024;17(1):1-14.
DOI: https://doi.org/10.14802/jmd.23216
Published online: November 21, 2023
1Department of Neurology, Changhua Christian Hospital, Changhua, Taiwan
2Department of Public Health, Chung Shan Medical University, Taichung, Taiwan
3Brain and Mind Centre, School of Medical Sciences, The University of Sydney, Camperdown, New South Wales, Australia
4Department of Electrical Engineering, National Changhua University of Education, Changhua, Taiwan
- Corresponding author: Shey-Lin Wu, MD, PhD Department of Neurology, Changhua Christian Hospital, 135 Nanhsiao Street, Changhua 500, Taiwan / Tel: +8864-7238595 / Fax: +8864-7232942 / E-mail: 14132@cch.org.tw
- Corresponding author: Simon J G Lewis, MD, PhD Brain and Mind Centre, School of Medical Sciences, The University of Sydney, Camperdown, New South Wales 2006, Australia / Tel: +61 2 9114 4121 / Fax: +61 2 9351 0855 / E-mail: profsimonlewis@gmail.com
Copyright © 2024 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,692 Views
- 139 Download
ABSTRACT
- Circadian disruption is being increasingly recognized as a critical factor in the development and progression of Parkinson’s disease (PD). This review aims to provide an in-depth overview of the relationship between circadian disruption and PD by exploring the molecular, cellular, and behavioral aspects of this interaction. This review will include a comprehensive understanding of how the clock gene system and transcription–translation feedback loops function and how they are diminished in PD. The article also discusses the role of clock genes in the regulation of circadian rhythms, as well as the impact of clock gene dysregulation on mitochondrial function, oxidative stress, and neuroinflammation, including the microbiota-gut-brain axis, which have all been proposed as being crucial mechanisms in the pathophysiology of PD. Finally, this review highlights potential therapeutic strategies targeting the clock gene system and circadian rhythm for the treatment of PD.
- Parkinson’s disease (PD) is a complex neurodegenerative disorder that has been traditionally characterized by its debilitating motor symptoms. However, recent research has provided information on significant nonmotor manifestations, among which circadian disruption emerges as a critical but underappreciated dimension [1]. The circadian clock, which is an intrinsic time-keeping system orchestrating physiological processes, appears to be altered and perturbed in PD. This scenario correspondingly contributes to sleep disturbances, cognitive decline, and possible exacerbation of motor symptoms [2]. This article explores the molecular and cellular underpinnings of this disruption by highlighting the roles of proteins, genes, and neurotransmitters at the intersection of circadian rhythms and PD pathology. Emerging evidence also links the gut microbiota-brain axis with circadian regulation and PD, thus suggesting a potentially transformative approach to our understanding of the etiology and progression of this disorder [3,4]. This new research direction emphasizes the implications of diagnostic strategies and therapeutic interventions; moreover, it advocates for a greater focus on the identification of reliable biomarkers, the development of personalized medicine, and the use of precision therapeutics from a holistic perspective of circadian disruption in PD. We believe that by integrating circadian health into the PD management framework, we can enhance our current therapeutic strategies and improve the quality of life of those individuals living with PD.
INTRODUCTION
- Circadian rhythm regulation
- Circadian rhythms are inherent timekeeping systems that govern a wide array of physiological processes, thus ensuring that they occur at biologically advantageous times. Spanning approximately 24 hours, these rhythms have evolved to align with the Earth’s rotation, thereby synchronizing the internal processes of an organism with the external environment. This internal timer is controlled by genes and synchronized by environmental signals, such as light and nutrition, to regulate physiological activities in every cell structure [5]. Indeed, the 2017 Nobel Prize in Physiology and Medicine, which was awarded to Rosbash, Hall, and Young, recognized their groundbreaking work on the molecular dynamics of this circadian rhythm and the relevance of circadian synchronization to health [5-11].
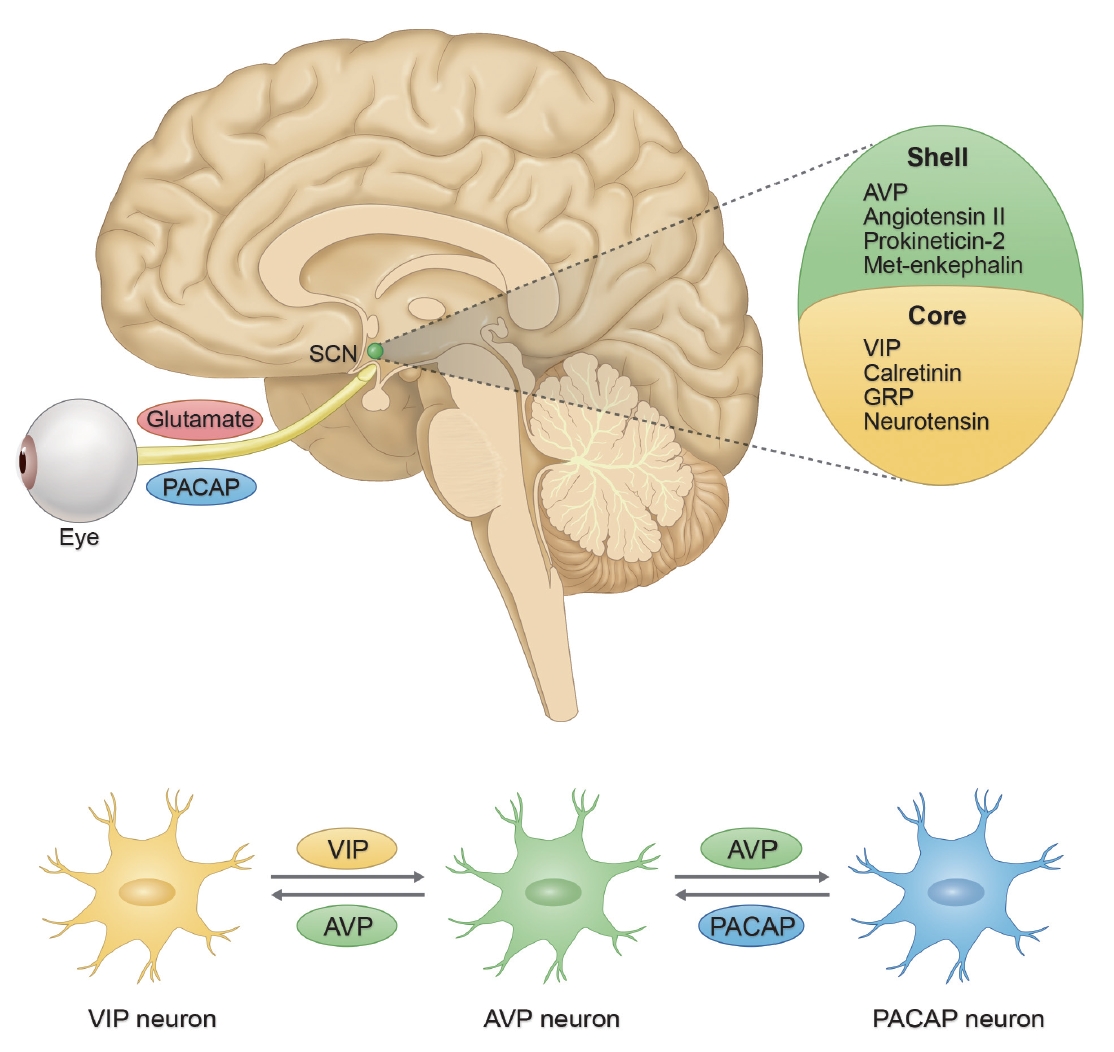
- A central idea of the orchestration of these rhythms in mammals is the suprachiasmatic nucleus (SCN), which is a dense cluster of neurons located in the anterior hypothalamus. The autonomy of the rhythmicity of the SCN is remarkable but not impervious to external cues. Such environmental signals, which are aptly known as “zeitgebers” (a German lexicon translating to “time givers”), rely predominantly on ambient light. The SCN receives direct input from the eyes through a pathway known as the retinohypothalamic tract. Specialized photoreceptive retinal ganglion cells containing pigment melanopsin absorb light and relay this information to the SCN. Neurons within the SCN exhibit rhythmic firing patterns, and these patterns are instrumental in conveying time-of-day information to various regions of the brain and peripheral tissues [12,13]. These patterns are supported by secondary input from structures such as the intergeniculate leaflet and brainstem [14].
- Without the influence of light, SCN neurons autonomously generate an intrinsic circadian rhythm, which produces an approximate 24-hour cycle. Harmonized output of the SCN is transmitted to peripheral molecular oscillators, thus extending its temporal influence throughout the organism [15]. Although these peripheral clocks possess innate rhythmic capabilities, their temporal alignment is orchestrated by the SCN via various modulatory pathways, including endocrine signaling, autonomic output, thermoregulatory changes, physical activity, and dietary patterns [16-19]. For example, rhythmic hormonal cascades originating from the hypothalamus and pituitary are driven by the SCN. Additionally, hormones such as melatonin, serotonin (5-HT), and glucocorticoids subsequently modulate circadian gene expression, thus establishing a pivotal feedback loop for circadian synchronization [20,21]. These hormonal mechanisms involve the secretion of specific neuropeptides and the intricate modulation of the hypothalamic‒pituitary‒adrenal axis, which subsequently influence the secretion of melatonin from the pineal gland, as well as secretion of glucocorticoids and catecholamines from the adrenal gland [22,23].
- Upon further investigation into the cellular architecture of the SCN, most of its neurons are GABAergic. Those neurons that reside in the ventrolateral core divisions of the SCN predominantly express neurotransmitters and neuropeptides, such as vasoactive intestinal polypeptide (VIP), calretinin, gastrin-related peptide, and neurotensin. In contrast, the divisions of the dorsomedial shell are enriched in neurons expressing arginine vasopressin (AVP), angiotensin II, prokineticin-2, and met-enkephalin [24]. A unique characteristic of SCN neurons is their intercellular coupling, which promotes autonomous circadian oscillations in both neuronal activity and gene expression. The VIP produced by ventrolateral core neurons plays a key role in this intercellular synchronization, whereby it influences other neuropeptides, such as AVP and gastrin-releasing peptide (GRP) (Figure 1). This intricate synchronization is crucial, and VIP knockout studies have demonstrated marked desynchronization of SCN activities [25-27].
- Recent studies have provided information on a subset of VIPpositive SCN neurons that display activity during dark periods, which is in contrast to the predominant pattern of SCN neuronal activity. These neurons have been postulated to play a pivotal role in modulating sleep patterns between activity bouts in nocturnal murines, either by inhibiting activity and fostering quiescence or via direct effects of sleep promotion [26,28]. This finding not only underscores the role of the SCN in maintaining 24- hour rhythms but also suggests its involvement in fine-tuning intricate features of the sleep–wake cycle.
- Molecular and cellular mechanisms
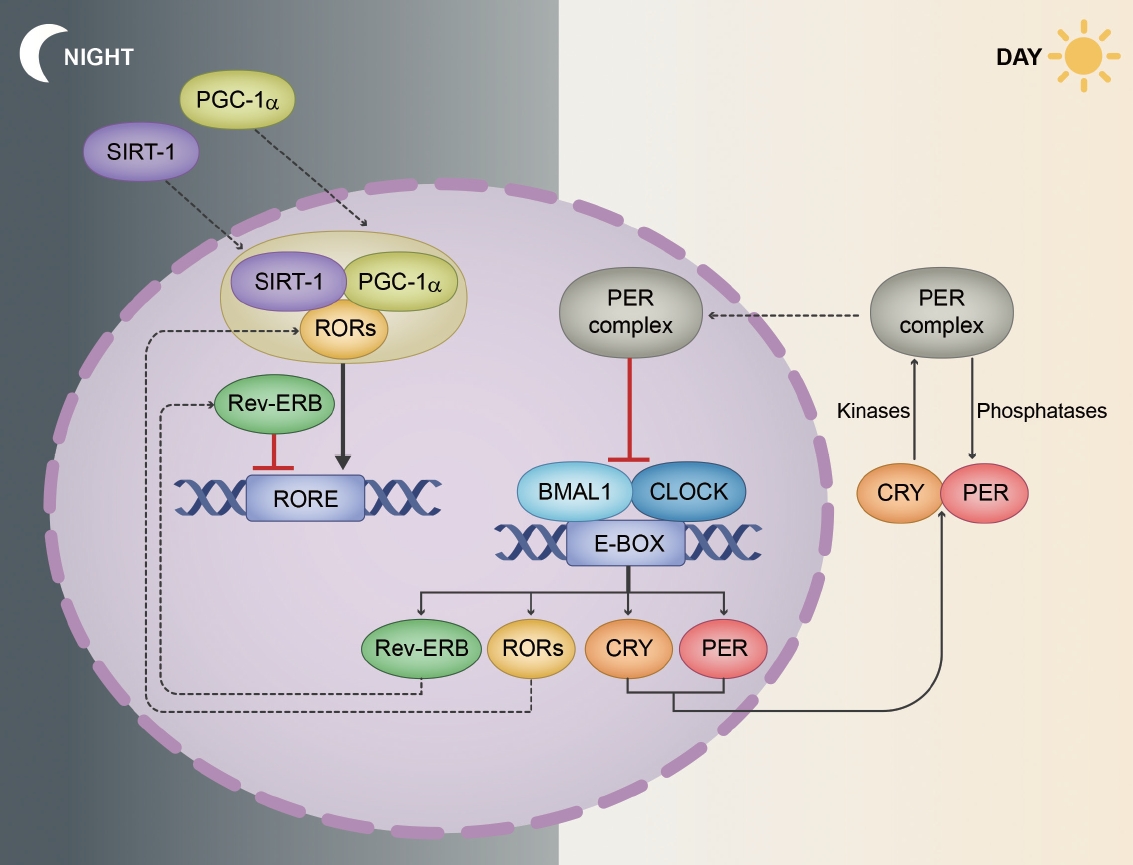
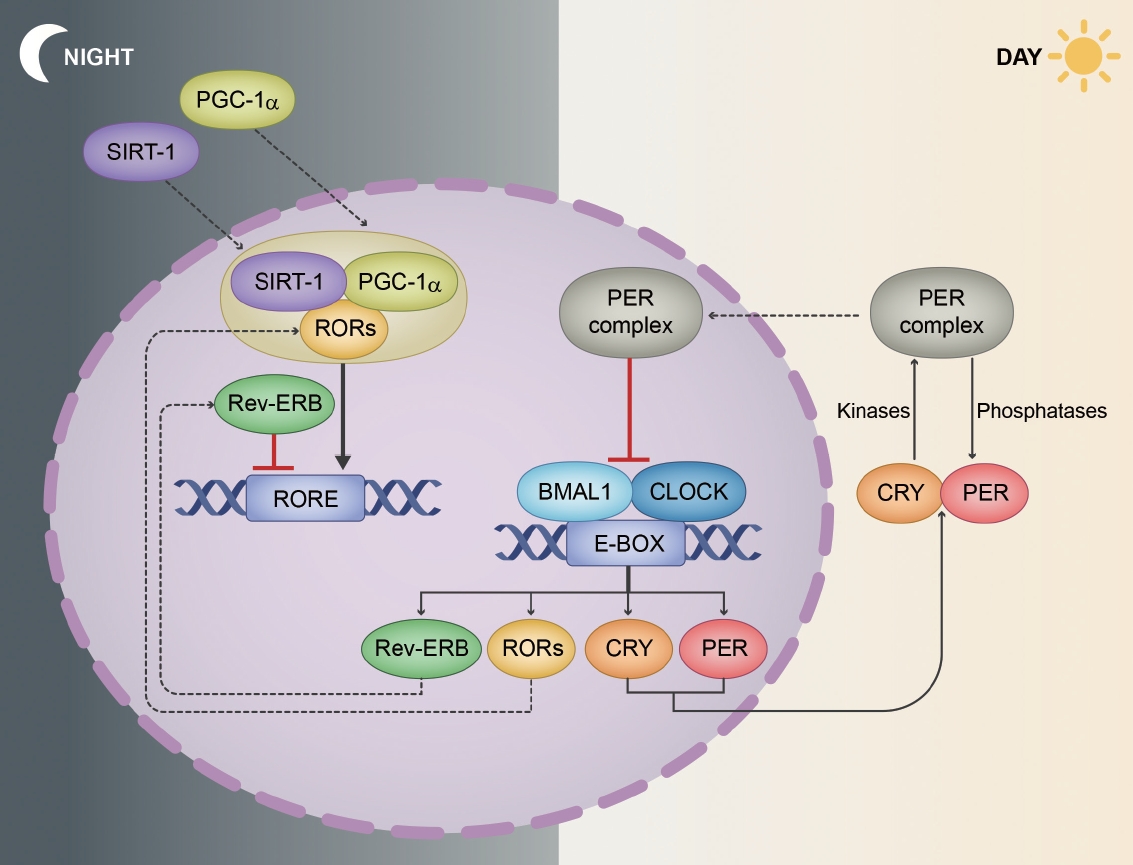
- The regulation of circadian rhythms at the molecular level is governed by a transcriptional/translational feedback loop (TTFL) [29]. Every major tissue in the mammalian body has rhythmic gene expression, and a substantial proportion of mammalian genes (ranging from 10% in rodents to more than 50% in primates [including humans]) exhibit rhythmic fluctuations that are tailored to specific tissue environments [30-32]. A key concept of this oscillation is the intricate feedback mechanism involving core circadian genes and their protein products. The synchronization of these rhythms across various tissues ensures the coordinated functioning of the body’s systems [24,33]. At the molecular level, circadian rhythms are maintained by a series of clock genes that are regulated by a TTFL (Figure 2) [34].
- The CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain and muscle ARNT-like 1) genes encode proteins that dimerize, thus forming the CLOCK-BMAL1 complex. This complex binds to enhancer box elements on the promoters of target genes, thus stimulating the transcription of downstream clock genes, especially Period (PER1, PER2, and PER3) and Cryptochrome (CRY1 and CRY2) genes [34,35].
- As the PER and CRY proteins accumulate in the cytoplasm, they form complexes and translocate to the nucleus. Herein, they function as negative regulators by inhibiting the activity of the CLOCK-BMAL1 complex. This results in decreased transcription of the PER and CRY genes, thus creating the negative feedback loop that defines the rhythm [35-37].
- The maintenance of a precise 24-hour cycle requires posttranslational modifications to fine-tune protein stability and function, with phosphorylation playing a pivotal role [38]. Kinases such as CK1δ/ε (casein kinase 1 delta/epsilon) phosphorylate PER proteins, thereby marking them for degradation. Moreover, phosphatases remove phosphate groups, thus stabilizing proteins. The interplay between kinases and phosphatases ensures timely protein degradation, which is crucial for the precision of the rhythm [38,39].
- Beyond the core TTFL, auxiliary feedback loops provide additional layers of regulation. For example, the CLOCK-BMAL1 complex also activates the transcription of the genes Rev-Erbα and retinoic acid receptor-related orphan receptor alpha (RORα). Rev-Erbα protein acts as a repressor by inhibiting BMAL1 transcription, whereas RORα enhances BMAL1 transcription by binding to ROR-responsive elements on the BMAL1 promoter. This loop intersects with the core TTFL, thus providing stability and robustness [40,41].
- Recent studies have provided more information on the interaction between cellular metabolism and the circadian clock. Nicotinamide adenine dinucleotide (NAD+) levels, which vary with the circadian rhythm, influence the activity of sirtuin 1 (SIRT1), which is an NAD-dependent deacetylase sirtuin-1. Importantly, SIRT1 can deacetylate BMAL1, thus affecting its stability and influencing the pace of the clock [42-44].
- Upon light exposure, the photopigment melanopsin is activated in retinal ganglion cells [45]. This activation influences intracellular signaling pathways that ultimately impact the levels of the PER2 protein, which helps to reset the clock [46].
- The precise coordination of the TTFL ensures that cells can anticipate and prepare for daily changes in their environment. This autonomous cell rhythm, when synchronized across billions of cells, ensures that tissues and organs function in harmony [34,47]. Moreover, several other genes, often termed “clock-controlled genes,” are regulated by the circadian rhythm, thus further amplifying the impact of TTFL on cell function. These genes govern a host of processes, ranging from metabolism to DNA repair, which emphasizes the widespread influence of the circadian system [48,49].
CIRCADIAN RHYTHM
Positive regulators
Negative regulators
Posttranslational modifications
Molecular interactions with light cues
- PD: beyond motor symptoms
- Although it is primarily diagnosed by its core motor features [50], it is well known that nonmotor symptoms are more prominent and bothersome to the patient’s quality of life, especially during the advanced stages of the disease (Table 1). In addition, many nonmotor features have been recognized during the prodromal period of the disease, including REM sleep behavior disorder, excessive daytime sleepiness, hyposmia, constipation, orthostatic hypotension, sexual dysfunction, anxiety, or depression [51], which are often not declared due to embarrassment or unawareness [52].
- Significantly, there is emerging evidence to suggest that certain sleep-related symptoms in PD are associated with circadian misalignment, which may represent a bidirectional relationship [53]. Two extensive cohort studies have recently indicated a potential link between disturbances in circadian rhythm and a higher likelihood of developing PD. Leng et al. [54] evaluated 2,930 community-dwelling men who were 65 years or older without PD at baseline, and subjects were observed for an 11-year period. Circadian parameters generated by wrist actigraphy-extended cosinor analysis (specifically, amplitude, mesor, and robustness) were found to be potent indicators of PD risk. Individuals in the lowest quartile for these circadian measures demonstrated an approximately threefold elevated risk of developing PD compared to those in the highest quartile [54]. Another expansive cohort study included 72,242 UK Biobank participants aged 37–73 years, wherein subjects were monitored for a median duration of 6.1 years. Circadian relative amplitude, which was derived from 7-day accelerometry data, served as a key measure to assess circadian rhythm disturbance. The study found that people with diminished relative amplitude exhibited increased risks in a range of neurological and psychiatric conditions, with risk ratios of 1.33 for PD in their fully adjusted models [55]. These findings highlight the substantial role of circadian disruption as a common risk factor for PD and underscore the prognostic significance of prodromal circadian markers concerning the onset of PD. Little is known about whether this disruption of the circadian system may impact mitochondrial dysfunction [56], oxidative stress [57], and neuroinflammation [58], which are all considered to be potential contributors to the neuropathology of PD.
- Behavioral and clinical evidence
- Sleep disturbance affects 60% to 98% of patients with PD, especially in the more advanced stages of the disease [59]. In addition to sleep disturbance, diurnal changes in other motor and nonmotor symptoms, such as the disruption of the rest-activity cycle, variation in blood pressure or cardiac rhythms, impaired sleep and alertness, and oscillations in mood, have also been associated with disease progression [60].
- Compared to healthy subjects, previous studies have suggested the relevance of PD and disruption of circadian rhythm via activity measurements [61,62]. Surprisingly, actigraphy recording rest-activity in PD has not demonstrated that lower activity and amplitude correlate with more advanced disease. However, such studies have also demonstrated a phase advance in PD, thus indicating a disturbance in circadian activity rhythm [63,64]. Moreover, diurnal variation in cardiovascular systems (reflected by increased blood pressure variability, reverse dip, and awakening hypotension) has also been reported in PD [65]. This clinical and preclinical evidence supports the assertion that circadian rhythm dysregulation may be a driver of the pathogenesis of PD [66].
- Molecular crosstalk between PD and circadian rhythm
- Although the exact causes of PD are still unknown, new evidence suggests that disturbances in circadian rhythm and clock gene expression may be involved in PD pathophysiology [67,68]. The intertwined nature of clock genes and TTFL in cellular regulation indicates that their disruption can have systemic effects. In PD, this has been observed through altered neurotransmitter release patterns (especially dopamine), disturbed sleep architecture, metabolic dysregulations, gastrointestinal disturbances, and even immune system abnormalities [69-73]. Clock gene disruptions can lead to misaligned dopamine release patterns, thus providing a window into the complex mechanisms underlying PD symptomatology [73,74]. Dopaminergic neurons, which represent the primary targets in PD, exhibit intrinsic circadian rhythms governed by the TTFL. Dysregulation of these clock genes has been observed in PD patients and animal models of PD (Table 2). McClung et al. [74] found that CLOCK mutant mice exhibited increased dopamine cell firing in the ventral tegmental area, thus suggesting that clock gene disruptions can directly affect dopaminergic function. Disturbances in the feedback loop within these neurons can also lead to changes in dopamine secretion patterns, thus contributing to the motor symptoms observed in PD [75,76]. These findings suggest a clear link between the clock gene system and the dopaminergic dysfunction observed in PD.
- Several studies have investigated the associations between clock genes and the different phenotypes observed in PD. A case‒control study in a Han Chinese population found that the ARNTL (BMAL1) and PER1 genes were associated with susceptibility to PD and specific phenotypes. The variant ARNTL (rs900147) showed a positive correlation with tremor-dominant (TD) cases, whereas PER1 (rs2225380) showed a positive correlation with postural instability and gait difficulty (PIGD) cases. The allele frequencies did not significantly differ between TD and PIGD, thus indicating no significant genetic variation between subtypes [77]. These findings suggest that clock genes could actually provide the foundation for the manifestation of specific PD symptoms.
- Clock gene variants have also been implicated in the disease phenotype, with the CLOCK T3111C variant found to be an independent risk factor for motor fluctuations and sleep disorders in Chinese PD patients [67,68,78,79]. Cai et al. [68] also reported that lower expression of the clock gene BMAL1 in PD patients during the dark period was associated with disease severity, thus suggesting that the extent of circadian rhythm disruption, as indicated by clock gene expression levels, may also serve as a severity marker in PD [68].
- In summary, these studies indicate that clock genes are associated with susceptibility to PD, specific phenotypes, motor fluctuations, sleep disorders, and disease severity.
- Circadian rhythms regulate the oscillations of tight junction proteins in the blood‒brain barrier (BBB); thus, disrupted circadian rhythms can lead to increased permeability of the BBB, altered expression of BBB transporters, and changes in the expression of tight junction proteins in the BBB [80,81]. The breakdown of BMAL1 has been shown to impair BBB integrity via pericyte dysfunction [82]. These findings suggest that disruption of circadian rhythms can directly affect BBB function, which could contribute to the development of PD [83].
- Beyond the disruptions that occur in the BBB, Willison et al. [84] proposed that circadian dysfunction may even accelerate the underlying pathology of PD by increasing oxidative stress and mitochondrial disruption. Indeed, the decomposition of BMAL1 expression has been shown to cause terminal synaptic damage, death of dopaminergic neurons, and aggravation of motor dysfunction in the MPTP-induced PD model [85]. Furthermore, it has been reported that the accumulation of α-synuclein can destabilize BMAL1 mRNA via miR-155, which can affect circadian rhythm [86]. Taken together, these results suggest that there is a bidirectional relationship between disruptions in the circadian clock system and the neuropathology of PD that, if better understood, could have implications for diagnosis and treatment.
- It has been suggested that clock genes not only regulate circadian rhythms but also play a significant role in neuroprotection through processes such as mitochondrial dysfunction, protein aggregation, neuroinflammation, and oxidative stress pathways (Figure 3) [79,85,87-90].
- Mitochondrial dysfunction is a prominent feature of the pathophysiology of PD, and emerging evidence suggests a link between clock gene dysregulation and mitochondrial function. The clock gene system regulates mitochondrial dynamics, including processes such as mitochondrial transport, fusion, and fission, as well as mitophagy, which is the selective degradation of damaged mitochondria [91]. Clock genes regulate mitochondrial dynamics, biogenesis, and oxidative phosphorylation via the modulation of key transcription factors, such as PGC-1α and NRF1 [92-94]. Thus, dysregulation of clock genes can disrupt mitochondrial homeostasis, thus leading to impaired energy production, increased oxidative stress, and neuronal dysfunction in PD [89,95].
- Oxidative stress, which results from an imbalance between reactive oxygen species (ROS) production and antioxidant defense mechanisms, is believed to be a key contributor to PD pathogenesis [57]. Clock genes play a crucial role in regulating redox homeostasis by modulating the expression of antioxidant enzymes and stress response genes [70]. Disruptions in clock gene expression can lead to increased ROS production and impaired antioxidant defense mechanisms, thus contributing to oxidative stress and neurodegeneration in PD [90,96,97]. Furthermore, dysfunction of the NAD-dependent deacetylase SIRT1, which is regulated by clock genes, is a hallmark of PD. SIRT1 is involved in maintaining cellular redox homeostasis, and its dysfunction may contribute to oxidative stress and neurodegeneration in PD [43,44,98].
- Neuroinflammation is a salient characteristic of PD, which is highlighted by the pronounced activation of microglia and the subsequent release of proinflammatory cytokines [99]. The immune system is synchronized with the circadian clock to ensure that immune responses are optimally timed to improve their efficacy [100]. Astrocytes possess intrinsic circadian clocks and release cytokines and chemokines, thus modulating the activity of surrounding neurons and glial cells [101,102]. Microglia, which are the resident immune cells of the brain, exhibit circadian patterns in their morphology and phagocytic activity [103,104]. Their role becomes crucial in neurodegenerative diseases where aberrant circadian rhythms can exacerbate disease progression. Within these cells, TTFL modulates several immune responses [85,105,106], and it is known that BMAL1 can inhibit the production of proinflammatory cytokines, whereas its disruption leads to heightened inflammatory responses [107,108]. Thus, the targeting of clock genes and their downstream inflammatory pathways may provide novel therapeutic approaches for mitigating neuroinflammation and slowing disease progression in PD [88].
- The gut-brain axis refers to the bidirectional communication between the gastrointestinal tract and the central nervous system, and it involves neural, hormonal, and immunological pathways [109]. Recent studies have demonstrated a bidirectional relationship between the gut microbiota and the clock gene system. Disruptions in the gut microbiota, such as dysbiosis or alterations in microbial metabolites, can lead to clock gene dysregulation and circadian rhythm disturbances [110-112]. In contrast, disruptions in circadian rhythm can also lead to gut dysfunction, such as altered gut motility, increased intestinal permeability, and dysregulated immune responses [113-115]. Thus, dysregulation of the gut-brain axis, which is mediated by circadian dysregulation, can further exacerbate neuroinflammation and neurodegeneration in PD [116].
- Other neurodegenerative disorders, such as Alzheimer’s disease (AD), Huntington’s disease, and amyotrophic lateral sclerosis, have been associated with disruptions in circadian rhythms [55,97,117-121]. These disruptions are not only considered manifestations of the diseases but may also directly contribute to their pathogenesis [122]. The role of circadian rhythm abnormalities in these disorders has become increasingly recognized, with evidence suggesting that circadian rhythm disruption and sleep disorders aggravate neurodegeneration; correspondingly, neurodegenerative diseases can disrupt circadian rhythms and sleep [123].
CIRCADIAN DISRUPTION IN PD
The role of CLOCK genes in disease risk, phenotype, and prognosis
Pathophysiological basis of CLOCK gene abnormalities in PD
The role of CLOCK genes beyond circadian rhythm
Clock gene dysregulation and mitochondrial dysfunction
Clock gene dysregulation and oxidative stress
Clock gene dysregulation and neuroinflammation
Clock gene dysregulation and the gut-brain axis
Clock gene dysregulation and other neurodegenerative disorders
- The early detection of circadian disruption can allow for the identification of prodromal PD, thus allowing for timely disease-modifying interventions. In fact, as highlighted above, it is possible that such strategies may even specifically attempt to restore circadian disruption in an effort to slow the pace of disease progression, which would represent a novel therapeutic strategy for the prevention and management of PD [54]. When considering the importance of circadian disruptions in PD, the future direction and challenge for circadian research in PD should focus on the identification of circadian biomarkers. In addition to traditional measurements of melatonin or cortisol levels [124,125], new approaches should focus on the evaluation of peripheral clock gene expression [2,126,127]. To date, proteomic studies of pathology related to circadian rhythm disorders have been performed with limited success; moreover, they lack high-quality cohort studies on the onset and course of PD [128-130]. Other chronobiological signatures obtained from wearable devices, such as actigraphy devices, combined with advanced bioinformatics tools to assess core body temperature and rhythm of rest-activity, may also offer noninvasive and efficient methods to assess PD onset or progression [131,132].
BIOMARKERS AND DIAGNOSTICS
- Along with understanding the role of circadian rhythm disruption in PD and facilitating research on the interplay between neurodegeneration and circadian rhythm disruption, there is a new perspective for therapeutic potential (Table 3) [133]. Some simple approaches already exist, such as the effect of high-intensity exercise, which not only improves sleep efficiency but also improves circadian rhythm [134]. Similarly, light therapy has already been explored in PD [135,136]. Despite the low cost, easy accessibility, and excellent safety profile, further studies are needed to clarify the optimal timing, appropriate duration, optimal illumination, and wavelength of the light itself [135].
- The antioxidative capabilities of melatonin [137] and its role in circadian synchronization [138] have positioned it as a potential neuroprotective and chronotherapeutic agent. A recent meta-analysis suggested that melatonin can significantly improve subjective sleep quality and total sleep time in PD with good safety and tolerability [139]. Emerging research has substantiated the efficacy of prolonged release melatonin formulations [140], as well as melatonin receptor agonists [141], in enhancing subjective sleep quality among patients diagnosed with PD. However, the endogenous circadian rhythm governing melatonin secretion exhibits interindividual variability and is susceptible to modulation by external variables, including dietary intake, physical activity, photic stimuli, and even dopaminergic medications [95,138,142-145]. Addressing these confounding factors may potentiate the efficacy of melatonin in the context of individualized therapeutic regimens.
- Recent advances in pharmacological research have led to the development of small-molecule modulators designed to target aberrant circadian systems. The CK1δ/ε inhibitor known as CKI-7 has been found to significantly reduce endogenous Aβ peptide [146], thus indicating its importance in neuroprotective strategies, such as those for AD. Furthermore, other small modulators inhibiting CDKs (cyclin-dependent kinases) or JNK (c-Jun N-terminal kinases) have period-lengthening activities because of their neuroprotective effects on CKIδ in some animal model studies [147,148]. One recent MPTP-induced PD preclinical study demonstrated some preservation of dopaminergic neurons and a partial restoration of striatal dopamine levels by using this approach [147].
- Rev-Erbα is a crucial negative regulator in the circadian clock system that regulates cellular circadian rhythms and energy metabolism and has been associated with the attenuation of neuroinflammation in PD pathology [149]. Thus, the potential therapeutic use of Rev-Erbα agonists (such as GSK4112) and antagonists (such as SR8278) to improve circadian dysregulation in neurodegenerative conditions has been suggested and requires further study [150]. Regardless of the agent that is evaluated, future treatments may rely on exploring the efficacy of chronotherapy, whereby medications need to be administered in synchronization with an individual’s biological rhythm to optimize their therapeutic effects and to minimize side effects [118].
THERAPEUTIC POTENTIAL OF CIRCADIAN RHYTHM REGULATION
- The role of circadian rhythm is just beginning to be understood in PD. There is clearly an intricate relationship between the clock gene system, the circadian rhythm, and the pathology underlying PD. A better understanding of the clock gene and TTFL disruptions in PD will potentially offer new therapeutic strategies. For example, molecular modulators, gene therapies, and even lifestyle interventions (such as controlled light exposure and diet) need to be explored more fully to realign disrupted circadian rhythms and potentially alter the course of the disease. The modulation of clock gene activity or the realignment of the TTFL can mitigate some of the symptoms or even slow the progression of PD. These insights pave the way for personalized therapeutic interventions and offer hope for better disease management.
CONCLUSION
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
SJGL is supported by an NHMRC Leadership Fellowship (#1195830).
-
Author Contributions
Conceptualization: Simon J G Lewis, Shey-Lin Wu. Data curation: Yen-Chung Chen, Wei-Sheng Wang. Formal analysis: Yen-Chung Chen, Wei- Sheng Wang. Funding acquisition: Simon J G Lewis. Investigation: Simon J G Lewis, Shey-Lin Wu, Yen-Chung Chen. Methodology: Yen-Chung Chen. Project administration: Simon J G Lewis, Shey-Lin Wu. Resources: all authors. Software: Yen-Chung Chen, Wei-Sheng Wang. Supervision: Simon J G Lewis, Shey-Lin Wu. Validation: Simon J G Lewis, Shey-Lin Wu. Visualization: Yen-Chung Chen. Writing—original draft: Yen-Chung Chen, Wei-Sheng Wang. Writing—review & editing: Simon J G Lewis, Shey-Lin Wu.
Notes

| Study |
Behavioral and physiological alternations |
|
|---|---|---|
| Presentation | Significance | |
| Sun et al., [151] 2019 | Sleep-awake behavior | Increased α-synuclein in CSF was noted in adults with chronic sleep apnea, supporting poor sleep may be related pathogenesis of Parkinson’s disease |
| Jiang et al., [152] 2023 | Sleep-awake behavior | Poor PD sleepers have severe non-motor symptoms; in addition, the increase of nocturnal arousal may predict the progression of motor symptoms |
| Brooks et al., [63] 2020 | Rest-activity rhythms | Continuous actigraphy can detect rest-activity disruption in PD, which is associated with motor severity and H&Y stage |
| Obayashi et al., [64] 2021 | Rest-activity rhythms | PD patients exhibited a phase advance in circadian activity rhythm, along with amplitude reduction |
| Vallelonga et al., [65] 2019 | Variations in cardiac rhythms or blood pressure | Patients with α-synucleinopathies showed a circadian rhythm disruption characterized by increased BP variability |
| Shen et al., [153] 2022 | Variations in cardiac rhythms or blood pressure | 24-hour ambulatory BP monitoring is an important method to evaluate the BP alterations in PD |
| Suzuki et al., [154] 2007 | Mood swings | PD patients with depression show an altered circadian rhythm in temperature |
| Study |
Molecular alternations: clock genes expression from human/animals |
|
| Gene/intervention | Phenotype | |
| Lee et al., [155] 2010 | BMAL1 | Alternation in rhythm of locomotor activity, premature aging, risk factor of cancer |
| Gu et al., [77] 2015 | BMAL1 | Tremor dominant subtype, contribution not only to circadian dysfunction but also PD pathogenesis |
| DeBruyne et al., [156] 2007 | CLOCK | Circadian disruption presenting in locomotor activity and response to light |
| Lou et al., [78] 2018 | CLOCK | An independent risk factor for motor fluctuations and sleep disturbance in PD |
| Hua et al., [157] 2012 | CRY1 | Besides circadian disruption, more prone to depression |
| Masubuchi et al., [158] 2005 | PER1 | Fail to adapt to environmental light-dark cycle |
| Gu et al., [77] 2015 | PER1 | Postural instability subtype, also contribution to circadian dysfunction and PD pathogenesis |
| Fu et al., [159] 2002 | PER2 | Caricadian control and tumor suppressor gene |
| Lou et al., [160] 2017 | PER2 | Regulation of psycho-behavioral control, hormone secretion, mood, and sleep |
| Study |
Molecular alternations: preclinical models from animals |
|
| Gene/intervention | Phenotype | |
| Tanaka et al., [161] 2012 | MPTP | Lengthen the circadian period of locomotor activity |
| Hayashi et al., [104] 2013 | MPTP | Alterations of clock genes expression |
| Choudhury and Daadi, [162] 2018 | MPTP | Experience PD-like motor and non-motor symptoms with circadian disruption |
| Franke et al., [163] 2016 | MPTP | Prodromal stage PD symptoms |
| Wang et al., [90] 2018 | 6-OHDA | Alterations of clock genes expression and antioxidant molecules |
| Yang et al., [164] 2021 | 6-OHDA | Variations in circadian rhythms of blood pressure and body temperature |
| Mattam and Jagota, [165] 2015 | Rotenone | Alterations of clock genes expression |
| Valadas et al., [166] 2018 | PARK | Sleep fragmentation and circadian dysregulation |
| Liu et al., [167] 2022 | LRRK2 | Lower clock gene expression and disrupted sleep-awake cycle with reduced REM, NREM and total sleep time |
| McDowell et al., [168] 2014 | α-synuclein | Produce sleep disruption with increased NREM sleep, decreased REM sleep and altered oscillatory EEG activity |
| Kudo et al., [169] 2011 | α-synuclein | The wheel-running activity shows reduced nighttime activity and increased fragmentation. |
| Liu et al., [86] 2023 | α-synuclein | Disrupts biorhythms by destabilizing BMAL1 mRNA through miR-155. |
| Langley et al., [170] 2021 | MitoPark | Display all-light- or all-dark-induced circadian rhythm dysfunction |
| Taylor et al., [171] 2009 | VMAT2-Deficient Model | A shorter latency to behavioral sleep |
| Potential therapy for CRD | Benefits | Drawbacks |
|---|---|---|
| Physical exercise (Schenkman et al., [172] 2018) | Improvement in motor symptoms | Concern of physical fitness |
| Providing cardiovascular benefits as well | Fear and risks of falling | |
| May arrest progression of PD | Musculoskeletal injuries | |
| Melatonin supplement (Videnovic et al., [124] 2014) | Improvement in sleep and poor alertness | Possible side effects, such as, headache, nausea, dizziness, drowsiness |
| Beneficial for the sleep-awake cycle | ||
| Antioxidant activity | ||
| Light therapy (Rutten et al., [173] 2012; Endo et al., [135] 2020) | Beneficial in non-motor symptoms, especially in sleep and mood disorder | Still lack of evidence in optimal light exposure, illumination and wavelength |
| Simple and convenient | ||
| Low cost | ||
| No concern of drug adverse effect | ||
| Potentials to restore circadian rhythm | ||
| Small chemical modulators (Wang et al., [147] 2004; Hu et al., [148] 2015) | Alleviates behavioral impairment | Lack of evidence in human studies |
| Neuroprotective effects of dopaminergic neuron | ||
| Chronotherapy (Fifel and Videnovic, [118] 2019; Asadpoordezaki et al., [133] 2023) | To optimize medication effect | Difficult to propose a standard circadian schedule |
| Low cost | ||
| No concern of drug adverse effect | ||
| Potentials to block the development of non-motor symptoms |
- 1. Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR; NMSS Validation Group. The impact of non-motor symptoms on healthrelated quality of life of patients with Parkinson’s disease. Mov Disord 2011;26:399–406.ArticlePubMedPDF
- 2. Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 2014;71:589–595.ArticlePubMedPMC
- 3. Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson Dis 2017;3:3.ArticlePDF
- 4. Teichman EM, O’Riordan KJ, Gahan CGM, Dinan TG, Cryan JF. When rhythms meet the blues: circadian interactions with the microbiota-gut-brain axis. Cell Metab 2020;31:448–471.ArticlePubMed
- 5. Botanische Forschungen des Alexanderzuges. Nature 1903;68:292–293.ArticlePDF
- 6. Bargiello TA, Jackson FR, Young MW. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 1984;312:752–754.ArticlePubMedPDF
- 7. Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 1990;343:536–540.ArticlePubMedPDF
- 8. Liu X, Zwiebel LJ, Hinton D, Benzer S, Hall JC, Rosbash M. The period gene encodes a predominantly nuclear protein in adult Drosophila. J Neurosci 1992;12:2735–2744.ArticlePubMedPMC
- 9. Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young MW. Double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 1998;94:83–95.ArticlePubMed
- 10. Vosshall LB, Price JL, Sehgal A, Saez L, Young MW. Block in nuclear localization of period protein by a second clock mutation, timeless. Science 1994;263:1606–1609.ArticlePubMed
- 11. Zehring WA, Wheeler DA, Reddy P, Konopka RJ, Kyriacou CP, Rosbash M, et al. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell 1984;39(2 Pt 1):369–376.ArticlePubMed
- 12. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002;295:1070–1073.ArticlePubMed
- 13. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002;295:1065–1070.ArticlePubMedPMC
- 14. Edelstein K, Amir S. The role of the intergeniculate leaflet in entrainment of circadian rhythms to a skeleton photoperiod. J Neurosci 1999;19:372–380.ArticlePubMedPMC
- 15. Sanchez REA, Kalume F, de la Iglesia HO. Sleep timing and the circadian clock in mammals: past, present and the road ahead. Semin Cell Dev Biol 2022;126:3–14.ArticlePubMed
- 16. Allada R, Bass J. Circadian mechanisms in medicine. N Engl J Med 2021;384:550–561.ArticlePubMedPMC
- 17. Astiz M, Heyde I, Fortmann MI, Bossung V, Roll C, Stein A, et al. The circadian phase of antenatal glucocorticoid treatment affects the risk of behavioral disorders. Nat Commun 2020;11:3593.ArticlePubMedPMCPDF
- 18. Tahara Y, Aoyama S, Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci 2017;67:1–10.ArticlePubMedPDF
- 19. Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol 2002;12:1574–1583.ArticlePubMed
- 20. Mistlberger RE, Antle MC, Glass JD, Miller JD. Behavioral and serotonergic regulation of circadian rhythms. Biol Rhythm Res 2000;31:240–283.Article
- 21. Vriend J, Reiter RJ. Melatonin feedback on clock genes: a theory involving the proteasome. J Pineal Res 2015;58:1–11.ArticlePubMedPDF
- 22. Kolbe I, Dumbell R, Oster H. Circadian clocks and the interaction between stress axis and adipose function. Int J Endocrinol 2015;2015:693204.ArticlePubMedPMCPDF
- 23. Boonen E, Berghe GV. Novel insights in the HPA-axis during critical illness. Acta Clin Belg 2014;69:397–406.ArticlePubMed
- 24. Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 2001;916(1-2):172–191.ArticlePubMed
- 25. Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 2005;8:476–483.ArticlePubMedPMCPDF
- 26. Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, et al. Disrupted circadian rhythms in VIP-and PHI-deficient mice. Am J Physiol-Regul Integr Comp Physiol 2003;285:R939–R949.ArticlePubMed
- 27. Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, et al. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 2002;109:497–508.ArticlePubMed
- 28. Jones JR, Simon T, Lones L, Herzog ED. SCN VIP neurons are essential for normal light-mediated resetting of the circadian system. J Neurosci 2018;38:7986–7995.ArticlePubMedPMC
- 29. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002;418:935–941.ArticlePubMedPDF
- 30. Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018;359:eaao0318.ArticlePubMedPMC
- 31. Sun L, Ma J, Turck CW, Xu P, Wang GZ. Genome-wide circadian regulation: a unique system for computational biology. Comput Struct Biotechnol J 2020;18:1914–1924.ArticlePubMedPMC
- 32. Wucher V, Sodaei R, Amador R, Irimia M, Guigó R. Day-night and seasonal variation of human gene expression across tissues. PLoS Biol 2023;21:e3001986. ArticlePubMedPMC
- 33. Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007;129:605–616.ArticlePubMedPMC
- 34. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 2017;18:164–179.ArticlePubMedPDF
- 35. Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001;30:525–536.ArticlePubMed
- 36. Saini R, Jaskolski M, Davis SJ. Circadian oscillator proteins across the kingdoms of life: structural aspects. BMC Biol 2019;17:13.ArticlePubMedPMCPDF
- 37. Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science 2011;332:1436–1439.ArticlePubMed
- 38. Brenna A, Albrecht U. Phosphorylation and circadian molecular timing. Front Physiol 2020;11:612510.ArticlePubMedPMC
- 39. Narasimamurthy R, Hunt SR, Lu Y, Fustin JM, Okamura H, Partch CL, et al. CK1δ/ε protein kinase primes the PER2 circadian phosphoswitch. Proc Natl Acad Sci U S A 2018;115:5986–5991.ArticlePubMedPMC
- 40. Ikeda R, Tsuchiya Y, Koike N, Umemura Y, Inokawa H, Ono R, et al. REV-ERBα and REV-ERBβ function as key factors regulating mammalian circadian output. Sci Rep 2019;9:10171.ArticlePubMedPMCPDF
- 41. Feng D, Lazar MA. Clocks, metabolism, and the epigenome. Mol Cell 2012;47:158–167.ArticlePubMedPMC
- 42. Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 2013;153:1448–1460.ArticlePubMedPMC
- 43. Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008;134:329–340.ArticlePubMedPMC
- 44. Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009;324:654–657.ArticlePubMedPMC
- 45. Dannerfjord AA, Brown LA, Foster RG, Peirson SN. Light input to the mammalian circadian clock. In: Brown SA, editor. Circadian clocks: methods and protocols. Vol 2130. New York: Humana; 2021:233–247.
- 46. Brenna A, Ripperger JA, Saro G, Glauser DA, Yang Z, Albrecht U. PER2 mediates CREB-dependent light induction of the clock gene Per1. Sci Rep 2021;11:21766.ArticlePubMedPMCPDF
- 47. Hastings MH, Maywood ES, Brancaccio M. The mammalian circadian timing system and the suprachiasmatic nucleus as its pacemaker. Biology (Basel) 2019;8:13.ArticlePubMedPMC
- 48. Ripperger JA, Albrecht U. Clock-controlled genes. Binder MD, Hirokawa N, Windhorst U. Encyclopedia of Neuroscience. Berlin: Springer; 2009:752–757.
- 49. Korenčič A, Košir R, Bordyugov G, Lehmann R, Rozman D, Herzel H. Timing of circadian genes in mammalian tissues. Sci Rep 2014;4:5782.PubMedPMC
- 50. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–1601.ArticlePubMed
- 51. Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA 2020;323:548–560.ArticlePubMed
- 52. Chaudhuri KR, Prieto‐Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, Tluk S, et al. The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord 2010;25:704–709.ArticlePubMed
- 53. Minakawa EN. Bidirectional relationship between sleep disturbances and Parkinson’s disease. Front Neurol 2022;13:927994.ArticlePubMedPMC
- 54. Leng Y, Blackwell T, Cawthon PM, Ancoli-Israel S, Stone KL, Yaffe K. Association of circadian abnormalities in older adults with an increased risk of developing Parkinson disease. JAMA Neurol 2020;77:1270–1278.ArticlePubMedPMC
- 55. Chen SJ, Deng YT, Li YZ, Zhang YR, Zhang W, Chen SD, et al. Association of circadian rhythms with brain disorder incidents: a prospective cohort study of 72242 participants. Transl Psychiatry 2022;12:514.ArticlePubMedPMCPDF
- 56. Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 2016;139(Suppl 1):216–231.ArticlePubMed
- 57. Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinson Dis 2013;3:461–491.Article
- 58. Moehle MS, West AB. M1 and M2 immune activation in Parkinson’s disease: foe and ally? Neuroscience 2015;302:59–73.ArticlePubMed
- 59. Comella CL. Sleep disorders in Parkinson’s disease: an overview. Mov Disord 2007;22(Suppl 17):S367–S373.ArticlePubMed
- 60. Videnovic A, Willis GL. Circadian system—a novel diagnostic and therapeutic target in Parkinson’s disease? Mov Disord 2016;31:260–269.ArticlePubMedPMCPDF
- 61. van Hilten JJ, Kabel JF, Middelkoop HA, Kramer CG, Kerkhof GA, Roos RA. Assessment of response fluctuations in Parkinson’s disease by ambulatory wrist activity monitoring. Acta Neurol Scand 1993;87:171–177.ArticlePubMed
- 62. Whitehead DL, Davies AD, Playfer JR, Turnbull CJ. Circadian rest‐activity rhythm is altered in Parkinson’s disease patients with hallucinations. Mov Disord 2008;23:1137–1145.ArticlePubMed
- 63. Brooks C, Shaafi Kabiri N, Mortazavi F, Auerbach S, Bonato P, Erb MK, et al. Variations in rest-activity rhythm are associated with clinically measured disease severity in Parkinson’s disease. Chronobiol Int 2020;37:699–711.ArticlePubMed
- 64. Obayashi K, Saeki K, Yamagami Y, Kurumatani N, Sugie K, Kataoka H. Circadian activity rhythm in Parkinson’s disease: findings from the PHASE study. Sleep Med 2021;85:8–14.ArticlePubMed
- 65. Vallelonga F, Di Stefano C, Merola A, Romagnolo A, Sobrero G, Milazzo V, et al. Blood pressure circadian rhythm alterations in alpha-synucleinopathies. J Neurol 2019;266:1141–1152.ArticlePubMedPDF
- 66. Lauretti E, Di Meco A, Merali S, Praticò D. Circadian rhythm dysfunction: a novel environmental risk factor for Parkinson’s disease. Mol Psychiatry 2017;22:280–286.ArticlePubMedPDF
- 67. Ding H, Liu S, Yuan Y, Lin Q, Chan P, Cai Y. Decreased expression of Bmal2 in patients with Parkinson’s disease. Neurosci Lett 2011;499:186–188.ArticlePubMed
- 68. Cai Y, Liu S, Sothern RB, Xu S, Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur J Neurol 2010;17:550–554.ArticlePubMed
- 69. Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron 2003;39:889–909.ArticlePubMed
- 70. Lananna BV, Musiek ES. The wrinkling of time: aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol Dis 2020;139:104832.ArticlePubMedPMC
- 71. Baldini F, Hertel J, Sandt E, Thinnes CC, Neuberger-Castillo L, Pavelka L, et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol 2020;18:62.ArticlePubMedPMCPDF
- 72. Willis GL, Kelly AM, Kennedy GA. Compromised circadian function in Parkinson’s disease: enucleation augments disease severity in the unilateral model. Behav Brain Res 2008;193:37–47.ArticlePubMed
- 73. Logan RW, Parekh PK, Kaplan GN, Becker-Krail DD, Williams WP 3rd, Yamaguchi S, et al. NAD+ cellular redox and SIRT1 regulate the diurnal rhythms of tyrosine hydroxylase and conditioned cocaine reward. Mol Psychiatry 2019;24:1668–1684.ArticlePubMedPDF
- 74. McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A 2005;102:9377–9381.ArticlePubMedPMC
- 75. Korshunov KS, Blakemore LJ, Trombley PQ. Dopamine: a modulator of circadian rhythms in the central nervous system. Front Cell Neurosci 2017;11:91.ArticlePubMedPMC
- 76. Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc Natl Acad Sci U S A 2006;103:6386–6391.ArticlePubMedPMC
- 77. Gu Z, Wang B, Zhang YB, Ding H, Zhang Y, Yu J, et al. Association of ARNTL and PER1 genes with Parkinson’s disease: a case-control study of Han Chinese. Sci Rep 2015;5:15891.ArticlePubMedPMCPDF
- 78. Lou F, Li M, Luo X, Ren Y. CLOCK 3111T/C variant correlates with motor fluctuation and sleep disorders in Chinese patients with Parkinson’s disease. Parkinsons Dis 2018;2018:4670380.ArticlePubMedPMCPDF
- 79. Marano M, Rosati J, Magliozzi A, Casamassa A, Rappa A, Sergi G, et al. Circadian profile, daytime activity, and the Parkinson’s phenotype: a motion sensor pilot study with neurobiological underpinnings. Neurobiol Sleep Circadian Rhythms 2023;14:100094.ArticlePubMedPMC
- 80. Zhang SL, Lahens NF, Yue Z, Arnold DM, Pakstis PP, Schwarz JE, et al. A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat Commun 2021;12:617.ArticlePubMedPMCPDF
- 81. Pan W, Kastin AJ. The blood-brain barrier: regulatory roles in wakefulness and sleep. Neuroscientist 2017;23:124–136.ArticlePubMedPDF
- 82. Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, et al. Disruption of Bmal1 impairs blood-brain barrier integrity via pericyte dysfunction. J Neurosci 2017;37:10052–10062.ArticlePubMedPMC
- 83. Cuddapah VA, Zhang SL, Sehgal A. Regulation of the blood-brain barrier by circadian rhythms and sleep. Trends Neurosci 2019;42:500–510.ArticlePubMedPMC
- 84. Willison LD, Kudo T, Loh DH, Kuljis D, Colwell CS. Circadian dysfunction may be a key component of the non-motor symptoms of Parkinson’s disease: insights from a transgenic mouse model. Exp Neurol 2013;243:57–66.ArticlePubMedPMC
- 85. Liu WW, Wei SZ, Huang GD, Liu LB, Gu C, Shen Y, et al. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson’s disease mouse model. FASEB J 2020;34:6570–6581.ArticlePubMedPDF
- 86. Liu JY, Xue J, Wang F, Wang YL, Dong WL. α-synuclein-induced destabilized BMAL1 mRNA leads to circadian rhythm disruption in Parkinson’s disease. Neurotox Res 2023;41:177–186.ArticlePubMedPDF
- 87. Jiménez-Delgado A, Ortiz GG, Delgado-Lara DL, González-Usigli HA, González-Ortiz LJ, Cid-Hernández M, et al. Effect of melatonin administration on mitochondrial activity and oxidative stress markers in patients with Parkinson’s disease. Oxid Med Cell Longev 2021;2021:5577541.PubMedPMC
- 88. Kou L, Chi X, Sun Y, Han C, Wan F, Hu J, et al. The circadian clock protein Rev-erbalpha provides neuroprotection and attenuates neuroinflammation against Parkinson’s disease via the microglial NLRP3 inflammasome. J Neuroinflammation 2022;19:133.PubMedPMC
- 89. Pacelli C, Rotundo G, Lecce L, Menga M, Bidollari E, Scrima R, et al. Parkin mutation affects clock gene-dependent energy metabolism. Int J Mol Sci 2019;20:2772.ArticlePubMedPMC
- 90. Wang Y, Lv D, Liu W, Li S, Chen J, Shen Y, et al. Disruption of the circadian clock alters antioxidative defense via the SIRT1-BMAL1 pathway in 6-OHDA-induced models of Parkinson’s disease. Oxid Med Cell Longev 2018;2018:4854732.ArticlePubMedPMCPDF
- 91. Thorne NJ, Tumbarello DA. The relationship of α-synuclein to mitochondrial dynamics and quality control. Front Mol Neurosci 2022;15:947191.PubMedPMC
- 92. Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet 2019;10:435.ArticlePubMedPMC
- 93. Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC1α integrates the mammalian clock and energy metabolism. Nature 2007;447:477–481.ArticlePubMedPDF
- 94. Ye P, Li W, Huang X, Zhao S, Chen W, Xia Y, et al. BMAL1 regulates mitochondrial homeostasis in renal ischaemia-reperfusion injury by mediating the SIRT1/PGC-1alpha axis. J Cell Mol Med 2022;26:1994–2009.PubMedPMC
- 95. Aranda-Martínez P, Fernández-Martínez J, Ramírez-Casas Y, RodríguezSantana C, Rusanova I, Escames G, et al. Chronodisruption and loss of melatonin rhythm, associated with alterations in daily motor activity and mitochondrial dynamics in parkinsonian zebrafish, are corrected by melatonin treatment. Antioxidants (Basel) 2023;12:954.ArticlePubMedPMC
- 96. Maiese K. Moving to the rhythm with clock (circadian) genes, autophagy, mTOR, and SIRT1 in degenerative disease and cancer. Curr Neurovasc Res 2017;14:299–304.ArticlePubMedPMC
- 97. Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 2013;123:5389–5400.ArticlePubMedPMC
- 98. Ribeiro RFN, Pereira D, de Almeida LP, Silva MMC, Cavadas C. SIRT1 activation and its circadian clock control: a promising approach against (frailty in) neurodegenerative disorders. Aging Clin Exp Res 2022;34:2963–2976.ArticlePubMedPDF
- 99. Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol 2022;22:657–673.ArticlePubMedPMCPDF
- 100. Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol 2018;18:423–437.ArticlePubMedPDF
- 101. Carver KA, Lourim D, Tryba AK, Harder DR. Rhythmic expression of cytochrome P450 epoxygenases CYP4x1 and CYP2c11 in the rat brain and vasculature. Am J Physiol Cell Physiol 2014;307:C989–C998.ArticlePubMedPMC
- 102. Wen S, Ma D, Zhao M, Xie L, Wu Q, Gou L, et al. Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat Neurosci 2020;23:456–467.ArticlePubMedPDF
- 103. Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 2015;45:171–179.ArticlePubMed
- 104. Hayashi Y, Koyanagi S, Kusunose N, Okada R, Wu Z, Tozaki-Saitoh H, et al. The intrinsic microglial molecular clock controls synaptic strength via the circadian expression of cathepsin S. Sci Rep 2013;3:2744.ArticlePubMedPMCPDF
- 105. Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, Herzog ED. Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Curr Biol 2017;27:1055–1061.ArticlePubMedPMC
- 106. Brancaccio M, Edwards MD, Patton AP, Smyllie NJ, Chesham JE, Maywood ES, et al. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 2019;363:187–192.ArticlePubMedPMC
- 107. Griffin P, Sheehan PW, Dimitry JM, Guo C, Kanan MF, Lee J, et al. REV-ERBα mediates complement expression and diurnal regulation of microglial synaptic phagocytosis. Elife 2020;9:e58765. ArticlePubMedPMCPDF
- 108. Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc Natl Acad Sci U S A 2018;115:E8460–E8468.ArticlePubMedPMC
- 109. Tan AH, Lim SY, Lang AE. The microbiome-gut-brain axis in Parkinson disease-from basic research to the clinic. Nat Rev Neurol 2022;18:476–495.ArticlePubMedPDF
- 110. Govindarajan K, MacSharry J, Casey PG, Shanahan F, Joyce SA, Gahan CG. Unconjugated bile acids influence expression of circadian genes: a potential mechanism for microbe-host crosstalk. PLoS One 2016;11:e0167319. ArticlePubMedPMC
- 111. Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep 2018;8:1395.ArticlePubMedPMCPDF
- 112. Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015;17:681–689.ArticlePubMedPMC
- 113. Weger BD, Gobet C, Yeung J, Martin E, Jimenez S, Betrisey B, et al. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab 2019;29:362–382.E8.ArticlePubMedPMC
- 114. Montagner A, Polizzi A, Fouché E, Ducheix S, Lippi Y, Lasserre F, et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016;65:1202–1214.ArticlePubMed
- 115. Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013;153:812–827.ArticlePubMed
- 116. Kline EM, Houser MC, Herrick MK, Seibler P, Klein C, West A, et al. Genetic and environmental factors in Parkinson’s disease converge on immune function and inflammation. Mov Disord 2021;36:25–36.PubMed
- 117. La Morgia C, Ross-Cisneros FN, Sadun AA, Carelli V. Retinal ganglion cells and circadian rhythms in Alzheimer’s disease, Parkinson’s disease, and beyond. Front Neurol 2017;8:162.ArticlePubMedPMC
- 118. Fifel K, Videnovic A. Chronotherapies for Parkinson’s disease. Prog Neurobiol 2019;174:16–27.ArticlePubMedPMC
- 119. Schurhoff N, Toborek M. Circadian rhythms in the blood-brain barrier: impact on neurological disorders and stress responses. Mol Brain 2023;16:5.ArticlePubMedPMCPDF
- 120. Malkani R, Attarian H. Sleep in neurodegenerative disorders. Curr Sleep Medicine Rep 2015;1:81–90.ArticlePDF
- 121. Voysey Z, Fazal SV, Lazar AS, Barker RA. The sleep and circadian problems of Huntington’s disease: when, why and their importance. J Neurol 2021;268:2275–2283.ArticlePubMedPDF
- 122. Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016;354:1004–1008.ArticlePubMedPMC
- 123. Shen Y, Lv QK, Xie WY, Gong SY, Zhuang S, Liu JY, et al. Circadian disruption and sleep disorders in neurodegeneration. Transl Neurodegener 2023;12:8.ArticlePubMedPMCPDF
- 124. Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 2014;71:463–469.ArticlePubMedPMC
- 125. Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, et al. Disturbances in melatonin secretion and circadian sleepwake regulation in Parkinson disease. Sleep Med 2014;15:342–347.ArticlePubMed
- 126. Fagotti J, Targa ADS, Rodrigues LS, Noseda ACD, Dorieux FWC, Scarante FF, et al. Chronic sleep restriction in the rotenone Parkinson’s disease model in rats reveals peripheral early-phase biomarkers. Sci Rep 2019;9:1898.ArticlePubMedPMCPDF
- 127. Requejo C, López-de-Ipiña K, Ruiz-Ortega JÁ, Fernández E, Calvo PM, Morera-Herreras T, et al. Changes in day/night activity in the 6-OHDA-induced experimental model of Parkinson’s disease: exploring prodromal biomarkers. Front Neurosci 2020;14:590029.ArticlePubMedPMC
- 128. Karayel O, Virreira Winter S, Padmanabhan S, Kuras YI, Vu DT, Tuncali I, et al. Proteome profiling of cerebrospinal fluid reveals biomarker candidates for Parkinson’s disease. Cell Rep Med 2022;3:100661.ArticlePubMedPMC
- 129. Bogetofte H, Jensen P, Okarmus J, Schmidt SI, Agger M, Ryding M, et al. Perturbations in RhoA signalling cause altered migration and impaired neuritogenesis in human iPSC-derived neural cells with PARK2 mutation. Neurobiol Dis 2019;132:104581.ArticlePubMed
- 130. Mondello S, Kobeissy F, Mechref Y, Zhao J, Talih FR, Cosentino F, et al. Novel biomarker signatures for idiopathic REM sleep behavior disorder: a proteomic and system biology approach. Neurology 2018;91:1117. Erratum for: Neurology 2018;91:e1710–e1715.
- 131. Wu JQ, Li P, Stavitsky Gilbert K, Hu K, Cronin‐Golomb A. Circadian rest‐activity rhythms predict cognitive function in early Parkinson’s disease independently of sleep. Mov Disord Clin Pract 2018;5:614–619.ArticlePubMedPMCPDF
- 132. Raupach AK, Ehgoetz Martens KA, Memarian N, Zhong G, Matar E, Halliday GM, et al. Assessing the role of nocturnal core body temperature dysregulation as a biomarker of neurodegeneration. J Sleep Res 2020;29:e12939. ArticlePubMedPDF
- 133. Asadpoordezaki Z, Coogan AN, Henley BM. Chronobiology of Parkinson’s disease: past, present and future. Eur J Neurosci 2023;57:178–200.ArticlePubMedPDF
- 134. Amara AW, Wood KH, Joop A, Memon RA, Pilkington J, Tuggle SC, et al. Randomized, controlled trial of exercise on objective and subjective sleep in Parkinson’s disease. Mov Disord 2020;35:947–958.ArticlePubMedPMCPDF
- 135. Endo T, Matsumura R, Tokuda IT, Yoshikawa T, Shigeyoshi Y, Node K, et al. Bright light improves sleep in patients with Parkinson’s disease: possible role of circadian restoration. Sci Rep 2020;10:7982.ArticlePubMedPMCPDF
- 136. Lin F, Su Y, Weng Y, Lin X, Weng H, Cai G, et al. The effects of bright light therapy on depression and sleep disturbances in patients with Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. Sleep Med 2021;83:280–289.ArticlePubMed
- 137. Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, et al. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res 2013;23:267–300.ArticlePubMedPDF
- 138. Lewy AJ, Emens J, Jackman A, Yuhas K. Circadian uses of melatonin in humans. Chronobiol Int 2006;23(1-2):403–412.ArticlePubMed
- 139. Ma H, Yan J, Sun W, Jiang M, Zhang Y. Melatonin treatment for sleep disorders in Parkinson’s disease: a meta-analysis and systematic review. Front Aging Neurosci 2022;14:784314.ArticlePubMedPMC
- 140. Ahn JH, Kim M, Park S, Jang W, Park J, Oh E, et al. Prolonged-release melatonin in Parkinson’s disease patients with a poor sleep quality: a randomized trial. Parkinsonism Relat Disord 2020;75:50–54.ArticlePubMed
- 141. De Berardis D, Fornaro M, Serroni N, Olivieri L, Marini S, Moschetta FS, et al. Agomelatine treatment of major depressive disorder in Parkinson’s disease: a case series. J Neuropsychiatry Clin Neurosci 2013;25:343–345.ArticlePubMed
- 142. Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, et al. Disturbances in melatonin secretion and circadian sleepwake regulation in Parkinson disease. Sleep Med 2014;15:342–347.ArticlePubMed
- 143. Nogueira LFR, Marqueze EC. Effects of melatonin supplementation on eating habits and appetite-regulating hormones: a systematic review of randomized controlled clinical and preclinical trials. Chronobiol Int 2021;38:1089–1102.ArticlePubMed
- 144. Kruk J, Aboul-Enein BH, Duchnik E. Exercise-induced oxidative stress and melatonin supplementation: current evidence. J Physiol Sci 2021;71:27.ArticlePubMedPMCPDF
- 145. Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms 1997;12:537–546.ArticlePubMedPDF
- 146. Flajolet M, He G, Heiman M, Lin A, Nairn AC, Greengard P. Regulation of Alzheimer’s disease amyloid-beta formation by casein kinase I. Proc Natl Acad Sci U S A 2007;104:4159–4164.PubMedPMC
- 147. Wang W, Shi L, Xie Y, Ma C, Li W, Su X, et al. SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Neurosci Res 2004;48:195–202.ArticlePubMed
- 148. Hu S, Cui W, Zhang Z, Mak S, Xu D, Li G, et al. Indirubin-3-oxime effectively prevents 6OHDA-induced neurotoxicity in PC12 cells via activating MEF2D through the inhibition of GSK3β. J Mol Neurosci 2015;57:561–570.ArticlePubMedPDF
- 149. Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007;318:1786–1789.ArticlePubMed
- 150. Chang YC, Kim JY. Therapeutic implications of circadian clocks in neurodegenerative diseases. J Neurosci Res 2020;98:1095–1113.ArticlePubMedPDF
- 151. Sun HL, Sun BL, Chen DW, Chen Y, Li WW, Xu MY, et al. Plasma α-synuclein levels are increased in patients with obstructive sleep apnea syndrome. Ann Clin Transl Neurol 2019;6:788–794.ArticlePubMedPMCPDF
- 152. Jiang Y, Chen Y, Li D, Zhu S, Gu R, Wang Y, et al. Sleep structure and related clinical characteristics in drug-naïve Parkinson’s disease with subjectively different sleep quality. Front Neurol 2023;14:1156910.ArticlePubMedPMC
- 153. Shen L, Yang X, Lu W, Chen W, Ye X, Wu D. 24-hour ambulatory blood pressure alterations in patients with Parkinson’s disease. Brain Behav 2022;12:e2428. ArticlePubMedPDF
- 154. Suzuki K, Miyamoto T, Miyamoto M, Kaji Y, Takekawa H, Hirata K. Circadian variation of core body temperature in Parkinson disease patients with depression: a potential biological marker for depression in Parkinson disease. Neuropsychobiology 2007;56:172–179.ArticlePubMedPDF
- 155. Lee Y, Lee J, Kwon I, Nakajima Y, Ohmiya Y, Son GH, et al. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J Cell Sci 2010;123:3547–3557.ArticlePubMedPDF
- 156. DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol 2007;17:R538–R539.ArticlePubMed
- 157. Hua P, Liu W, Kuo SH, Zhao Y, Chen L, Zhang N, et al. Association of Tef polymorphism with depression in Parkinson disease. Mov Disord 2012;27:1694–1697.ArticlePubMedPMCPDF
- 158. Masubuchi S, Kataoka N, Sassone-Corsi P, Okamura H. Mouse Period1 (mPER1) acts as a circadian adaptor to entrain the oscillator to environmental light/dark cycles by regulating mPER2 protein. J Neurosci 2005;25:4719–4724.ArticlePubMedPMC
- 159. Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002;111:41–50.ArticlePubMed
- 160. Lou F, Li M, Ren Y, Luo XG, Liu N, Li X. CLOCK rs1801260 polymorphism is associated with susceptibility to Parkinson’s disease in a Chinese population. Neurosci Bull 2017;33:734–736.ArticlePubMedPMCPDF
- 161. Tanaka M, Yamaguchi E, Takahashi M, Hashimura K, Shibata T, Nakamura W, et al. Effects of age-related dopaminergic neuron loss in the substantia nigra on the circadian rhythms of locomotor activity in mice. Neurosci Res 2012;74:210–215.ArticlePubMed
- 162. Choudhury GR, Daadi MM. Charting the onset of Parkinson-like motor and non-motor symptoms in nonhuman primate model of Parkinson’s disease. PLoS One 2018;13:e0202770. ArticlePubMedPMC
- 163. Franke SK, van Kesteren RE, Wubben JA, Hofman S, Paliukhovich I, van der Schors RC, et al. Progression and recovery of Parkinsonism in a chronic progressive MPTP-induction model in the marmoset without persistent molecular and cellular damage. Neuroscience 2016;312:247–259.ArticlePubMed
- 164. Yang S, Wan Y, Wu N, Song L, Liu Z, Zhao J, et al. L-3,4-dihydroxyphenylalanine recovers circadian rhythm disturbances in the rat models of Parkinson’s disease by regulating the D1R-ERK1/2-mTOR pathway. Front Aging Neurosci 2021;13:719885.ArticlePubMedPMC
- 165. Mattam U, Jagota A. Daily rhythms of serotonin metabolism and the expression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkinson’s disease male Wistar rat model and effect of melatonin administration. Biogerontology 2015;16:109–123.ArticlePubMedPDF
- 166. Valadas JS, Esposito G, Vandekerkhove D, Miskiewicz K, Deaulmerie L, Raitano S, et al. ER lipid defects in neuropeptidergic neurons impair sleep patterns in Parkinson’s disease. Neuron 2018;98:1155–1155.ArticlePubMed
- 167. Liu X, Yu H, Wang Y, Li S, Cheng C, Al-Nusaif M, et al. Altered motor performance, sleep EEG, and Parkinson’s disease pathology induced by chronic sleep deprivation in Lrrk2G2019S mice. Neurosci Bull 2022;38:1170–1182.ArticlePubMedPMCPDF
- 168. McDowell KA, Shin D, Roos KP, Chesselet MF. Sleep dysfunction and EEG alterations in mice overexpressing alpha-synuclein. J Parkinsons Dis 2014;4:531–539.ArticlePubMed
- 169. Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol 2011;232:66–75.ArticlePubMed
- 170. Langley MR, Ghaisas S, Palanisamy BN, Ay M, Jin H, Anantharam V, et al. Characterization of nonmotor behavioral impairments and their neurochemical mechanisms in the MitoPark mouse model of progressive neurodegeneration in Parkinson’s disease. Exp Neurol 2021;341:113716.ArticlePubMedPMC
- 171. Taylor TN, Caudle WM, Shepherd KR, Noorian A, Jackson CR, Iuvone PM, et al. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci 2009;29:8103–8113.ArticlePubMedPMC
- 172. Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de bovo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol 2018;75:219–226.ArticlePubMed
- 173. Rutten S, Vriend C, van den Heuvel OA, Smit JH, Berendse HW, van der Werf YD. Bright light therapy in Parkinson’s disease: an overview of the background and evidence. Parkinsons Dis 2012;2012:767105.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite