Articles
- Page Path
- HOME > J Mov Disord > Volume 17(1); 2024 > Article
-
Original Article
Emotion Recognition in Multiple System Atrophy: An Exploratory Eye-Tracking Study -
Victoria Sidoroff1
 , Federico Carbone1
, Federico Carbone1 , Philipp Ellmerer1, Stefanie Bair1, Alexandra Hoffmann2
, Philipp Ellmerer1, Stefanie Bair1, Alexandra Hoffmann2 , Thomas Maran3,4
, Thomas Maran3,4 , Florian Krismer1
, Florian Krismer1 , Philipp Mahlknecht1
, Philipp Mahlknecht1 , Katherina Mair1, Cecilia Raccagni1,5
, Katherina Mair1, Cecilia Raccagni1,5 , Jean-Pierre Ndayisaba1, Klaus Seppi1
, Jean-Pierre Ndayisaba1, Klaus Seppi1 , Gregor K. Wenning1
, Gregor K. Wenning1 , Atbin Djamshidian1
, Atbin Djamshidian1

-
Journal of Movement Disorders 2024;17(1):38-46.
DOI: https://doi.org/10.14802/jmd.23090
Published online: September 26, 2023
1Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
2Department of Psychology, University of Innsbruck, Innsbruck, Austria
3Department of Strategic Management & Leadership, University of Innsbruck, Innsbruck Austria
4Entrepreneurship and Innovation, Free University of Bozen-Bolzano, Bozen-Bolzano, Italy
5Department of Neurology, Provincial Hospital of Bolzano Teaching Hospital of Paracelsus Medical Private University Bolzano-Bozen, Bolzano, Italy
- Corresponding author: Atbin Djamshidian, MD, PhD Department of Neurology, Medical University of Innsbruck, Anichstrasse 35, Innsbruck 6020, Austria / Tel: +43-51250424279 / E-mail: atbin.djamshidian-tehrani@i-med.ac.at
Copyright © 2024 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,133 Views
- 89 Download
ABSTRACT
-
Objective
- Emotional processing is a core feature of social interactions and has been well studied in patients with idiopathic Parkinson’s disease (PD), albeit with contradictory results. However, these studies excluded patients with atypical parkinsonism, such as multiple system atrophy (MSA). The objective of this exploratory study was to provide better insights into emotion processing in patients with MSA using eye tracking data.
-
Methods
- We included 21 MSA patients, 15 PD patients and 19 matched controls in this study. Participants performed a dynamic and a static emotion recognition task, and gaze fixations were analyzed in different areas of interest. Participants underwent neuropsychological testing and assessment of depression and alexithymia.
-
Results
- MSA patients were less accurate in recognizing anger than controls (p = 0.02) and had overall fewer fixations than controls (p = 0.001). In the static task, MSA patients had fewer fixations (p < 0.001) and a longer time to first fixation (p = 0.026) on the eye region. Furthermore, MSA patients had a longer fixation duration overall than PD patients (p = 0.004) and longer fixations on the nose than controls (p = 0.005). Alexithymia scores were higher in MSA patients compared to controls (p = 0.038).
-
Conclusion
- This study demonstrated impaired recognition of anger in MSA patients compared to HCs. Fewer and later fixations on the eyes along with a center bias suggest avoidance of eye contact, which may be a characteristic gaze behavior in MSA patients.
- Multiple system atrophy (MSA) is a relentlessly progressive neurodegenerative disease, with patients exhibiting a poor response to levodopa [1]. Typically, these patients have a higher disease burden and more motor complications than patients with idiopathic Parkinson’s disease (PD) [2,3]. Aside from reduced levodopa responses of parkinsonism and autonomic failure, patients suffer from nonmotor symptoms, including cognitive impairment and executive dysfunction [4]. Ocular abnormalities such as blepharospasm, square-wave jerks, hypometria of saccades and an impaired vestibular-ocular reflex are common in patients with atypical parkinsonism, including MSA [5]. Furthermore, previous studies have shown that a detailed analysis of eye movements may be a useful method to measure cognitive function [6]. Eye tracking, including systematic measurement of saccades and fixations, may therefore be a useful tool for assessing cognitive flexibility. In MSA patients, studies have shown longer latencies in pro-saccade and anti-saccade tasks compared to PD patients, suggesting a different gaze pattern which may also be a promising biomarker to distinguish between these disorders [7].
- In addition to use in saccadic paradigms, eye tracking can also be used to assess emotion recognition. Impairment in both expressing and recognizing emotions may lead to difficulties in social interaction and communication. Deficits in emotion processing have been described in patients with basal ganglia dysfunction, such as Huntington’s disease and PD [8]. More specifically, PD patients had a different scanning behavior with more fixations in the right visual hemifield and impaired recognition of anger and surprise compared to healthy controls (HCs) [9]. Moreover, PD patients with mild cognitive impairment were less accurate at recognizing anger than PD patients without cognitive impairment and HCs [10]. However, studies in PD patients have reported conflicting results, and the influences of many confounding factors, such as the impact of hypomimia or levodopa therapy, remain unclear [11,12]. Moreover, to the best of our knowledge, no study has assessed gaze behavior in face exploration and emotion recognition in MSA patients. To date, studies in patients with MSA have shown a blunted affect [13] and profound deficits in theory of mind tasks, particularly in affective versions of the task [14]. Imaging studies have shown damage in the temporal region, which is important for cognition [15], and dysfunction in the cingulum, which plays a key role in emotion processing and social cognition [16]. In line with this, emotional incontinence is common in MSA patients, probably due to alterations in the cortico-limbic-subcortico-thalamic-ponto-cerebellar network [17,18].
- Thus, the aim of the study was to assess emotion processing in patients with MSA and directly compare the results of PD patients and HCs using eye tracking data. Given the aforementioned findings, we hypothesized that we would observe impaired emotion recognition in both patient groups compared to HCs and that MSA patients would have distinct gaze patterns from HCs that may also differ from those in PD patients.
INTRODUCTION
- In this prospective, single-center pilot study, we included 21 patients with clinically probable (n = 13) or possible (n = 8) MSA of either the parkinsonian (n = 15) or cerebellar type (n = 6), according to the Gilman diagnostic criteria.19 We also included 15 PD patients and 19 HCs who had equal baseline parameters (Table 1).
- Only participants without dementia, according to cognitive assessments, and those without sedative medication or antidepressant use were included. The study was reviewed and approved by the Ethics Committee of the Medical University of Innsbruck, Austria (Approval No. AN1979 336/4.19 401/5.10 [4464a]). All procedures performed in studies involving human participants were in accordance with the 2013 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all the patients included in the study.
- For each participant, all study procedures were performed on the same day in the same order. After written informed consent was obtained, demographic data and current medication use were recorded. Cognition was assessed either by the Montreal Cognitive Assessment (MoCA) [20] or the Mini Mental State Examination (MMSE) [21]. For adequate comparison, MoCA scores were converted to MMSE scores with a validated algorithm for the interconversion developed by van Steenoven et al. [22] using an equipercentile equating method for conversion. Cutoffs of MoCA scores > 22 and MMSE scores > 23 were used to exclude participants with dementia [23]. Wherever available, MoCA subscores of executive function (sum of scores on the Trail Making Test, phonemic fluency and verbal abstraction) were calculated [24]. To assess signs of alexithymia, the Toronto Alexithymia Scale (TAS-20) was administered, and a score of 52–60 was defined as the threshold for possible alexithymia, while a score greater than 60 was defined as the threshold for alexithymia [25]. There are three subscales in the TAS-20: 1) difficulty describing feelings, 2) difficulty identifying emotions, and 3) externally oriented thinking. Furthermore, every participant completed the Hospital Anxiety and Depression Scale (HADS-D) to assess signs of depression or anxiety, which may influence emotional processing. To estimate motor disease severity, the Movement Disorder Society revision of the Unified PD Rating Scale (MDS-UPDRS) was administered to MSA and PD patients [26].
- All participants performed two facial emotion recognition tasks using an eye tracker (Tobii TX‐300 eye tracker with Tobii Pro Studio Software 3.4.8.1348; https://www.tobii.com/products/software?creative=641444153221&keyword=eye%20 tracking%20software&matchtype=p&network=g&device=c& gclid=CjwKCAjwv-2pBhB-EiwAtsQZFPsiHweE3HvWSBH6S1mHKv_eE4sjG2GWjeWt45N29w2_Q3ulP7TXgRoCCeQQAvD_BwE). A 23” screen at a viewing distance of 64 cm was used to present the stimuli. The tasks were performed in a quiet, windowless room to avoid possible distractions or interactions. The eye tracker recorded every eye position on the screen, and software algorithms summarized the raw data into different events, such as saccades and fixations. A fixation is defined as a stop in eye movement to acquire visual information, and saccades are used to move the eyes from one point to another. In our study, we use a cutoff of 50 ms to distinguish between a fixation (> 50 ms) and a saccade (< 50 ms), as the cutoff varies among studies [27]. Before recording, an initial nine-point calibration was performed, where participants had to follow a red dot with their eyes. To avoid a systematic center bias, participants had to fixate on a decentralized fixation cross prior to every stimulus for 2 seconds. All stimuli were then displayed on the screen in the same order and duration for every participant. A screen with all possible responses appeared after the stimulus, and the participants indicated their choice verbally to the experimenter, who recorded the reaction time by clicking the left mouse button and writing down the answer.
- The modified Geneva Emotion Recognition Test (emotion recognition dynamic task; ER-D) includes 24 3,000 ms video clips presenting male and female actors demonstrating eight different emotions [28]. Participants have to choose between the primary emotions of joy, anger, fear and disgust and the secondary emotions of surprise, amusement, pride and despair.
- The second task consisted of 63 faces selected from the NimStim-Set of Emotional Facial Expression Pictures (emotion recognition static task; ER-S) [29]. Photos of faces expressing three different emotions (joy, anger and fear) are shown for 6,000 ms. Every emotion is displayed at three different intensities (100% vs. 66% vs. 33%) by morphing the expression. Along with these pictures, faces showing a neutral expression are included. To avoid ceiling effects with too many correct answers, participants are asked to choose among neutral, joy, anger, fear, disgust, surprise or despair; this choice includes more options than the emotions displayed.
- For both tasks, the eye tracker detected fixations in predefined areas of interest (AOIs; whole face, eye region, mouth and nose). The face region included all other AOIs. Eye-tracking parameters, including the time to first fixation, fixation count and total fixation duration, were exported. If an emotion was shown multiple times during one test, mean values were calculated for all parameters.
- Statistical analyses were carried out with SPSS (version 27.0.1.0; IBM Corp., Armonk, NY, USA). Mean values of eye-tracking parameters were calculated for every AOI separately. Post hoc analysis of separate emotions was performed if the face AOI reached a significance level of p < 0.05. Emotions were either analyzed separately or clustered into positive (joy, amusement and pride) and negative (anger, fear, disgust, and despair) groups. Surprise and neutral faces were analyzed separately. A time to first fixation < 0.1 sec was excluded, as this implied noncompliance in fixating on the decentralized cross before exploring the face. Depending on the distribution, either parametric (independent sample t‐tests) or nonparametric (Mann–Whitney U or Kruskal–Wallis tests) analyses were used to compare demographic and eye-tracking parameters. Analysis of correlations between eye-tracking parameters and scale scores was carried out using Spearman’s correlation analysis. Post hoc comparisons were corrected using Bonferroni correction.
MATERIALS & METHODS
- The demographic data are summarized in Table 1. Aside from higher disease severity than PD patients (p < 0.001), MSA patients had the overall highest HADS-D scores, followed by PD patients and HCs (13.4 vs. 8.6 vs. 5.5; p < 0.001). Regarding the TAS-20 score, 10% of MSA patients fulfilled the criteria for alexithymia, while 19% of MSA patients, 10% of controls and 7% of PD patients had possible alexithymia. Analyses of the bivariate correlations of HADS scores and TAS-20 scores with emotion recognition and eye-tracking parameters did not reveal a significant influence of either score. MoCA subscores of executive function did not differ between MSA patients and controls.
- Emotion recognition–dynamic test
- On the ER-D task, the three groups exhibited similar recognition rates (p = 0.236) (Table 1). Pairwise comparisons revealed a lower accuracy for recognizing anger in MSA patients than in HCs (p = 0.016), without a difference between MSA-P and MSAC or between possible and prodromal MSA. Further analysis indicated an inverse correlation of TAS part III scores with recognition of negative emotions (τ = -0.285; p = 0.038). There was no correlation between MoCA subscores of executive function and emotion recognition.
- Regarding eye-tracking parameters, there were no significant differences in the overall time to first fixation and total fixation duration in each AOI among the groups (p > 0.1). There was, however, a significant group difference in the fixation count within the whole face throughout all emotions (p = 0.002). Post hoc analyses revealed that MSA patients had lower fixation counts than HCs (6.7 vs. 8.1; p = 0.001) (Table 2). Analysis of fixation counts for negative emotions revealed that MSA patients had lower counts than HCs (p = 0.003) but not PD patients (p = 0.08); there was no difference between PD patients and HCs (p > 0.1). When assessing positive emotions, MSA patients had fewer fixation counts in the face region (p = 0.01) than HCs. There were no other group differences (all p values > 0.1). Detailed eye-tracking parameters are displayed in Table 2.
- Bivariate correlation showed significant correlations of MSA duration with face fixation counts (τ = -0.492; p = 0.023) and eye fixation counts (τ = -0.490; p = 0.024). The levodopa equivalent daily dose (LEDD) inversely correlated with fixation counts in the face region (τ = -0.548; p = 0.01) in MSA patients. Comparison of the reaction time (from stimulus onset to participant response) did not show any differences among the three groups.
- Emotion recognition–static test
- In the ER-S, the three groups exhibited similar recognition rates (p = 0.66) (Table 1), without differences according to emotion type or intensity. There were no significant correlations with age, sex, HADS scores or TAS-20 scores. The reaction time (from stimulus onset to participant response) did not differ among the groups, and within-group comparisons of the first and second halves of the test did not show an overall change in reaction time (p > 0.1).
- When further analyzing the eye-tracking parameters (Table 3), the overall time to first fixation did not show significant differences among the three groups (p > 0.1). However, the time to first fixation in the eye region showed a significant difference among the groups (p = 0.031), and post hoc analysis revealed a significantly longer time to first fixation in the eye region in MSA patients than in HCs (p = 0.026); there were no other group differences. MSA patients had the longest total fixation duration on the whole face (p = 0.005; MSA vs. PD: p = 0.004), nose (p = 0.006; MSA vs. HC: p = 0.005) and mouth; in contrast, they had the shortest total fixation duration on the eye region compared to PD patients and HCs.
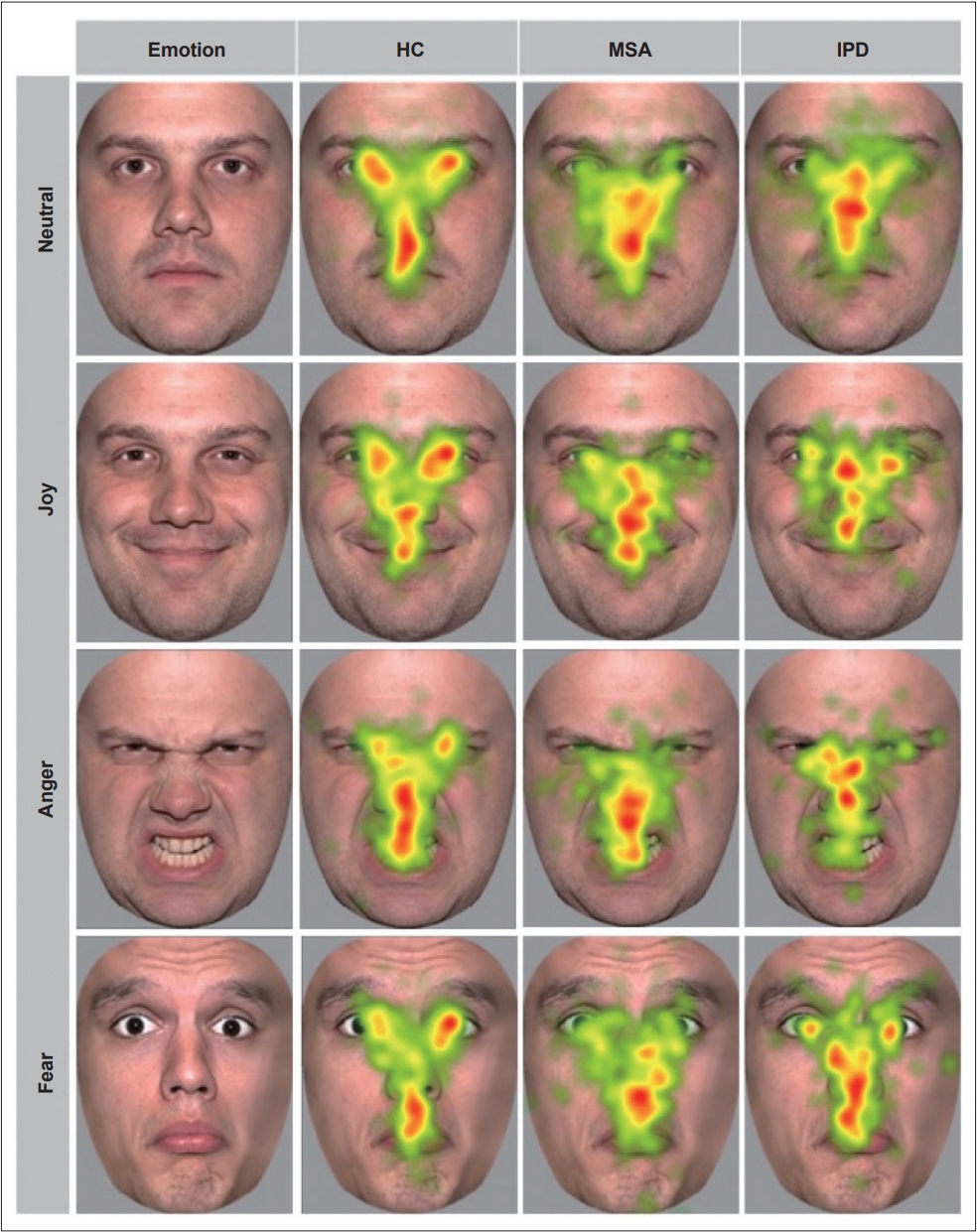
- Both MSA and PD patients had significantly fewer fixations in the face region than HCs (p < 0.001; MSA vs. HC: p < 0.001, PD vs. HC: p = 0.029), as well as fewer fixations in the eye region than HCs (p < 0.001). These results were observed for positive, negative and neutral faces. There were no significant differences in fixation counts on the nose and mouth among the three groups (p > 0.1). To ensure that the lower fixation counts in both patient groups and the slower times to first fixation were not due to fatigue, we compared the total fixations in the first half to those in the second half of the ER-S; we found no difference (p > 0.9). A heatmap of overall fixations among the groups is shown in Figure 1.
RESULTS
- In this study, we assessed emotion recognition and gaze behavior in patients with MSA and PD and compared their results to those of HCs. We found that MSA patients exhibited less accurate anger recognition than HCs in the dynamic task. Although the reason for this impairment remains unclear, it is likely that the poorer response to dopaminergic therapy and the more advanced neurodegenerative phenotype with greater motor disability in our MSA group compared to our PD group may have played an important role. Previous studies have reported a selective disruption of anger recognition due to dopaminergic antagonism in HCs with intact recognition of all other emotions [30]. Moreover, unmedicated PD patients or those with withdrawal of dopamine replacement therapy exhibit an impairment of anger recognition [8,31,32]. It has been hypothesized that dysfunction of ventral striatal dopamine release and impairment of dopamine projections to the ventral striatum, the orbitofrontal cortex and the anterior cingulate cortex may be responsible for this selective deficit in emotion recognition [33,34]. In addition, anger recognition deficits have also been linked to cerebellar volume loss [35]. Along with degeneration of the substantia nigra and the striatum, degeneration of the cerebellum is typical in MSA [36] and may be responsible for these anger deficits.
- Alexithymia scores were also significantly higher in MSA patients than in HCs, which is a novel finding. In particular, the subscores for identifying emotions were significantly lower in MSA patients than in PD patients and controls. A deficit in emotional experience and expression has been reported across multiple psychiatric and neurological disorders [37-40]. These impairments are associated with reduced quality of life and an increased caregiver burden in PD [41]. A recent study assessing patients with PD, frontotemporal dementia and progressive supranuclear palsy reported higher alexithymia scores in 80% of patients than in HCs [42]. Furthermore, a recent study on patients with restless legs syndrome and addictive behavior showed similar results, with increased difficulties in describing feelings and identifying emotions compared to HCs [43]. Higher alexithymia scores have also been linked to poorer emotion recognition, consistent with our results.
- In addition, we found fewer overall fixations and a center bias in MSA patients compared to PD patients and HCs. This is in line with a recent study on patients with PD, MSA and progressive supranuclear palsy that showed a reduction in macro saccades and a strong center bias in these patients compared to HCs [44]. Although the reason for this center bias remains unclear, it is possible that increased anxiety as well as higher alexithymia scores may be responsible for this gaze pattern in our MSA cohort. Previous studies have also demonstrated a center bias with avoidance of eye contact in patients with anxiety disorders [45] and in those with alexithymia [46,47]. The eye and mouth regions provide the most information for recognizing faces and emotions, while the nose region has the lowest contribution to recognizing known patterns [48]. Although MSA patients tend to focus more on the nose, they exhibit similar recognition of most emotions to the other groups, implying that even a shorter exploration of helpful areas such as the eyes and mouth yields comparable results.
- In our study, MSA patients (but not PD patients) had longer times to first fixation and fewer fixations within the eye region than HCs. One could argue that this gaze behavior is the result of an oculomotor deficit due to disruption of subcortical and cortical saccadic eye movement control. However, the overall time to first fixation did not differ among the groups, and thus, this difference may be due to dysfunction of emotion processing. A previous study described reduced eye contact in patients with frontotemporal dementia compared to HCs [49]. Considering the lower MoCA scores in our MSA and PD groups, our results are in line with those of Waldthaler et al. [10], who showed fewer overall fixations in PD patients with mild cognitive impairment, hypothesizing that visuospatial deficits may lead to impaired selection of appropriate targets for fixations, thereby leading to a wider scanning area [10]. In contrast to a previous study [50], we did not find frontal executive dysfunction in our MSA patients. Therefore, the exact mechanism of the altered gaze patterns remains unclear.
- One aspect that should also be mentioned is that there were no significant differences in emotion recognition or gaze patterns between PD patients and HCs. Although previous studies have reported inconsistent results, this may be due to variation in the inclusion criteria, as our patients were matched in age with the HCs, had symptoms well controlled with levodopa, and were without cognitive impairment.
- We also want to highlight a potential limitation of our study. As MSA is a rare disease, the sample size in this study was small, and many individuals only fulfilled the diagnostic criteria for possible MSA. Although there was no difference between probable and possible MSA patients in terms of performance, the possibility of incorrect diagnoses cannot be ruled out. Additionally, the overall poor performance in terms of emotion recognition and the small sample size indicate that further study of this patient population is needed. Furthermore, we found poorer anger recognition in only the dynamic emotion recognition task, not in the static task. The PD patients were also older (although this difference was not significant), and sex was not properly matched among the groups. Furthermore, the impaired cognition and increased anxiety of our MSA patients may be confounding factors for our analysis, as anxiety may influence cognitive-emotional status, emotion recognition and saccadic velocity.
- Conclusion
- In summary, we demonstrated for the first time that patients with MSA had higher alexithymia scores than HCs and found differences in gaze patterns between MSA and PD patients. Our exploratory study identified selective deficits in anger recognition in MSA patients, which may be due to impaired cognition and increased anxiety, but reduced dopaminergic sensitivity or possible dysfunction of the cortico-limbic-subcorticothalamic-ponto-cerebellar network might also play a role.
DISCUSSION
-
Conflicts of Interest
Florian Krismer: none concerning this project. F.K. reports personal fees from Institut de Recherches Internationales Servier, Clarion Healthcare, the Austrian Society of Neurology and grant support from the MSA Coalition.
Klaus Seppi: none concerning this project. K.S. reports personal fees from Teva, UCB, Lundbeck, AOP Orphan Pharmaceuticals AG, Roche, Grünenthal and Abbvie, honoraria from the International Parkinson and Movement Disorders Society, research grants from FWF Austrian Science Fund, Michael J. Fox Foundation, and International Parkinson and Movement Disorder Society, outside the submitted work.
Gregor K. Wenning: none concerning this project. G.W. reports personal fees from Biohaven, Theravance, UCB, Lundbeck, and Ono, honoraria from the Austrian Autonomic Society, research grants from FWF Austrian Science Fund, International Parkinson and Movement Disorder Society, and US MSA Coalition, outside of the submitted work.
Atbin Djamshidian: none concerning this project. A.D. reports personal fees from Novo Nordisk, Roche, Grünenthal, Bial, Esai and Abbvie.
The above conflicts of interests does not affected to publish this manuscript. All remaining authors have declared no conflicts of interest.
-
Funding Statement
None
-
Author contributions
Conceptualization: Victoria Sidoroff, Atbin Djamshidian. Data curation: Alexandra Hoffmann. Formal analysis: Victoria Sidoroff, Atbin Djamshidian. Investigation: Victoria Sidoroff, Stefanie Bair. Methodology: Victoria Sidoroff, Federico Carbone, Philipp Ellmerer, Stefanie Bair, Cecilia Raccagni, Gregor K. Wenning, Atbin Djamshidian. Project administration: Atbin Djamshidian. Resources: Victoria Sidoroff, Federico Carbone, Philipp Ellmerer, Florian Krismer, Philipp Mahlknecht, Katherina Mair, Jean-Pierre Ndayisaba, Klaus Seppi, Gregor K. Wenning. Software: Alexandra Hoffmann, Thomas Maran. Supervision: Atbin Djamshidian. Validation: Alexandra Hoffmann, Thomas Maran. Visualization: Victoria Sidoroff. Writing—original draft: Victoria Sidoroff. Writing—review & editing: Federico Carbone, Philipp Ellmerer, Stefanie Bair, Alexandra Hoffmann, Thomas Maran, Florian Krismer, Philipp Mahlknecht, Katherina Mair, Cecilia Raccagni, Jean-Pierre Ndayisaba, Klaus Seppi, Gregor K. Wenning, Atbin Djamshidian.
Notes
- We thank all participants to take their time and be part of this project.
Acknowledgments
| MSA | IPD | HC |
p-value |
||||
|---|---|---|---|---|---|---|---|
| Total | MSA/IPD | MSA/HC | IPD/HC | ||||
| Sex (male:female) | 7:14 | 10:5 | 7:12 | 0.110* | 0.15 | > 0.999 | 0.25 |
| Age, yr | 60.3 ± 7.7 | 64.4 ± 8.8 | 60.5 ± 6.5 | 0.169† | 0.37 | > 0.999 | 0.46 |
| Disease duration, month | 14 (4, 30.5) | 48 (36, 108) | - | - | 0.005‡ | - | - |
| LEDD | 400 (0, 502) | 310 (300, 600) | - | - | 0.485‡ | - | - |
| MDS-UPDRS total | 73.7 ± 19.9 | 38.7 ± 19.1 | - | - | < 0.001§ | - | - |
| Hoehn & Yahr | 3 (3, 3) | 2 (2, 2) | - | - | < 0.001‡ | - | - |
| MoCA | 27.5 (25, 28) | 26 (26, 27) | 29 (27, 30) | 0.003ǁ | 0.107 | 0.035 | 0.002 |
| HADS-D | 13.4 ± 5.9 | 8.6 ± 5.3 | 5.5 ± 3.1 | < 0.001† | 0.018 | < 0.001 | 0.24 |
| TAS-20 | 46.2 ± 12.1 | 39.2 ± 11.5 | 37.6 ± 9.5 | 0.048† | 0.21 | 0.06 | > 0.999 |
| Subscore (1) | 14.3 ± 6.2 | 11.9 ± 5.2 | 10.1 ± 3.4 | 0.048 | 0.51 | 0.045 | 0.99 |
| Subscore (2) | 12.6 ± 4.6 | 9.1 ± 2.7 | 9.3 ± 3.1 | 0.009 | 0.026 | 0.024 | > 0.999 |
| Subscore (3) | 19.3 ± 5.8 | 18.2 ± 6.0 | 18.1 ± 6.1 | 0.793 | > 0.999 | > 0.999 | > 0.999 |
| ER-D (% right) | 50.4 ± 12.1 | 49,2 ± 12.9 | 55 ± 7.9 | 0.236† | > 0.999 | 0.53 | 0.36 |
| ER-S (% right) | 50.6 ± 6.8 | 52.9 ± 5.1 | 51.9 ± 8.7 | 0.736† | > 0.999 | > 0.999 | 0.76 |
Quantitative values are given in mean ± standard deviation or median and interquartile range.
* chi-squared test;
† one-way ANOVA;
‡ Mann-Whitney U Test;
§ Student’s t-Test;
ǁ Kruskal-Wallis Test.
MSA, multiple system atrophy; IPD, idiopathic Parkinson’s disease; HC, healthy controls; LEDD, levodopa equivalent dose; MDS-UPDRS, Movement Disorder Society revision of the Unified Parkinson’s Disease Rating Scale; MoCA, Montreal Cognitive Assessment; HADS-D, Hospital Anxiety and Depression Scale; TAS-20, Toronto Alexithymia scale; ER-D, emotion recognition dynamic task; ER-S, emotion recognition static task.
- 1. Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med 2015;372:1375–1376.Article
- 2. Chelban V, Catereniuc D, Aftene D, Gasnas A, Vichayanrat E, Iodice V, et al. An update on MSA: premotor and non-motor features open a window of opportunities for early diagnosis and intervention. J Neurol 2020;267:2754–2770.ArticlePubMedPMCPDF
- 3. Krismer F, Wenning GK. Multiple system atrophy: insights into a rare and debilitating movement disorder. Nat Rev Neurol 2017;13:232–243.ArticlePubMedPDF
- 4. Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG, et al. Cognitive impairment in multiple system atrophy: a position statement by the neuropsychology task force of the MDS multiple system atrophy (MODIMSA) study group. Mov Disord 2014;29:857–867.ArticlePubMedPMCPDF
- 5. Armstrong RA. Visual signs and symptoms of multiple system atrophy. Clin Exp Optom 2014;97:483–491.ArticlePubMed
- 6. White OB, Fielding J. Cognition and eye movements: assessment of cerebral dysfunction. J Neuroophthalmol 2012;32:266–273.PubMed
- 7. Brooks SH, Klier EM, Red SD, Mehta ND, Patel SS, Chuang AZ, et al. Slowed prosaccades and increased antisaccade errors as a potential behavioral biomarker of multiple system atrophy. Front Neurol 2017;8:261.ArticlePubMedPMC
- 8. Sprengelmeyer R, Young AW, Mahn K, Schroeder U, Woitalla D, Büttner T, et al. Facial expression recognition in people with medicated and unmedicated Parkinson’s disease. Neuropsychologia 2003;41:1047–1057.ArticlePubMed
- 9. Clark US, Neargarder S, Cronin-Golomb A. Visual exploration of emotional facial expressions in Parkinson’s disease. Neuropsychologia 2010;48:1901–1913.ArticlePubMedPMC
- 10. Waldthaler J, Krüger-Zechlin C, Stock L, Deeb Z, Timmermann L. New insights into facial emotion recognition in Parkinson’s disease with and without mild cognitive impairment from visual scanning patterns. Clin Park Relat Disord 2019;1:102–108.ArticlePubMedPMC
- 11. Bek J, Poliakoff E, Lander K. Measuring emotion recognition by people with Parkinson’s disease using eye-tracking with dynamic facial expressions. J Neurosci Methods 2020;331:108524.ArticlePubMed
- 12. Argaud S, Vérin M, Sauleau P, Grandjean D. Facial emotion recognition in Parkinson’s disease: a review and new hypotheses. Mov Disord 2018;33:554–567.ArticlePubMedPMCPDF
- 13. Fetoni V, Soliveri P, Monza D, Testa D, Girotti F. Affective symptoms in multiple system atrophy and Parkinson’s disease: response to levodopa therapy. J Neurol Neurosurg Psychiatry 1999;66:541–544.ArticlePubMedPMC
- 14. Santangelo G, Cuoco S, Picillo M, Erro R, Squillante M, Volpe G, et al. Theory of mind in multiple system atrophy: comparison with Parkinson’s disease and healthy subjects. J Neural Transm (Vienna) 2020;127:915–923.ArticlePubMedPDF
- 15. Caso F, Canu E, Lukic MJ, Petrovic IN, Fontana A, Nikolic I, et al. Cognitive impairment and structural brain damage in multiple system atrophy-parkinsonian variant. J Neurol 2020;267:87–94.ArticlePubMedPDF
- 16. Apps MA, Rushworth MF, Chang SW. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 2016;90:692–707.ArticlePubMedPMC
- 17. King RR, Reiss JP. The epidemiology and pathophysiology of pseudobulbar affect and its association with neurodegeneration. Degener Neurol Neuromuscul Dis 2013;3:23–31.PubMedPMC
- 18. Rabins PV, Arciniegas DB. Pathophysiology of involuntary emotional expression disorder. CNS Spectr 2007;12(4 Suppl 5):17–22.ArticlePubMed
- 19. Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676.ArticlePubMedPMC
- 20. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699.ArticlePubMed
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198.PubMed
- 22. van Steenoven I, Aarsland D, Hurtig H, Chen-Plotkin A, Duda JE, Rick J, et al. Conversion between mini-mental state examination, Montreal cognitive assessment, and dementia rating scale-2 scores in Parkinson’s disease. Mov Disord 2014;29:1809–1815.ArticlePubMedPMC
- 23. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–935.ArticlePubMed
- 24. Donovan M, Mario C, Laurie G, Susan R, Chris T, Michael T. Chapter 13. Psychiatric rating scales. In: Aminoff M, Boller F, Swaab D, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier; 2012:227–237.
- 25. Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto alexithymia scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res 1994;38:23–32.ArticlePubMed
- 26. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170.ArticlePubMedPDF
- 27. Negi S, Mitra R. Fixation duration and the learning process: an eye tracking study with subtitled videos. J Eye Mov Res 2020;13:1.ArticlePubMedPMCPDF
- 28. Schlegel K, Grandjean D, Scherer KR. Introducing the Geneva emotion recognition test: an example of Rasch-based test development. Psychol Assess 2014;26:666–672.ArticlePubMed
- 29. Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009;168:242–249.ArticlePubMedPMC
- 30. Lawrence AD, Calder AJ, McGowan SW, Grasby PM. Selective disruption of the recognition of facial expressions of anger. Neuroreport 2002;13:881–884.ArticlePubMed
- 31. Lawrence AD, Goerendt IK, Brooks DJ. Impaired recognition of facial expressions of anger in Parkinson’s disease patients acutely withdrawn from dopamine replacement therapy. Neuropsychologia 2007;45:65–74.ArticlePubMed
- 32. Dujardin K, Blairy S, Defebvre L, Krystkowiak P, Hess U, Blond S, et al. Subthalamic nucleus stimulation induces deficits in decoding emotional facial expressions in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2004;75:202–208.PubMedPMC
- 33. Calder AJ, Keane J, Lawrence AD, Manes F. Impaired recognition of anger following damage to the ventral striatum. Brain 2004;127(Pt 9):1958–1969.ArticlePubMed
- 34. Lin CY, Tien YM, Huang JT, Tsai CH, Hsu LC. Degraded impairment of emotion recognition in Parkinson’s disease extends from negative to positive emotions. Behav Neurol 2016;2016:9287092.ArticlePubMedPMCPDF
- 35. Scharmüller W, Ille R, Schienle A. Cerebellar contribution to anger recognition deficits in Huntington’s disease. Cerebellum 2013;12:819–825.ArticlePubMedPMCPDF
- 36. Yang H, Wang N, Luo X, Lv H, Liu H, Li Y, et al. Cerebellar atrophy and its contribution to motor and cognitive performance in multiple system atrophy. Neuroimage Clin 2019;23:101891.ArticlePubMedPMC
- 37. Ridout N, Smith J, Hawkins H. The influence of alexithymia on memory for emotional faces and realistic social interactions. Cogn Emot 2021;35:540–558.ArticlePubMed
- 38. Suslow T, Günther V, Hensch T, Kersting A, Bodenschatz CM. Alexithymia is associated with deficits in visual search for emotional faces in clinical depression. Front Psychiatry 2021;12:668019.ArticlePubMedPMC
- 39. Lane RD, Sechrest L, Reidel R, Weldon V, Kaszniak A, Schwartz GE. Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosom Med 1996;58:203–210.ArticlePubMed
- 40. Assogna F, Cravello L, Orfei MD, Cellupica N, Caltagirone C, Spalletta G. Alexithymia in Parkinson’s disease: a systematic review of the literature. Parkinsonism Relat Disord 2016;28:1–11.ArticlePubMed
- 41. Klietz M, Schnur T, Drexel SC, Lange F, Paracka L, Huber MK, et al. Alexithymia is associated with reduced quality of life and increased caregiver burden in Parkinson’s disease. Brain Sci 2020;10:401.ArticlePubMedPMC
- 42. Sturm VE, Levenson RW. Alexithymia in neurodegenerative disease. Neurocase 2011;17:242–250.ArticlePubMedPMC
- 43. Ellmerer P, Heim B, Stefani A, Peball M, Werkmann M, Holzknecht E, et al. Augmentation in restless legs syndrome: an eye tracking study on emotion processing. Ann Clin Transl Neurol 2020;7:1620–1627.ArticlePubMedPMCPDF
- 44. Habibi M, Oertel WH, White BJ, Brien DC, Coe BC, Riek HC, et al. Eye tracking identifies biomarkers in α-synucleinopathies versus progressive supranuclear palsy. J Neurol 2022;269:4920–4938.ArticlePubMedPMCPDF
- 45. Schulze L, Renneberg B, Lobmaier JS. Gaze perception in social anxiety and social anxiety disorder. Front Hum Neurosci 2013;7:872.ArticlePubMedPMC
- 46. Bird G, Press C, Richardson DC. The role of alexithymia in reduced eyefixation in autism spectrum conditions. J Autism Dev Disord 2011;41:1556–1564.ArticlePubMedPDF
- 47. Fujiwara E. Looking at the eyes interferes with facial emotion recognition in alexithymia. J Abnorm Psychol 2018;127:571–577.ArticlePubMed
- 48. Davies G, Ellis H, Shepherd J. Cue saliency in faces as assessed by the “Photofit” technique. Perception 1977;6:263–269.ArticlePubMedPDF
- 49. Sturm VE, McCarthy ME, Yun I, Madan A, Yuan JW, Holley SR, et al. Mutual gaze in Alzheimer’s disease, frontotemporal and semantic dementia couples. Soc Cogn Affect Neurosci 2011;6:359–367.ArticlePubMedPMC
- 50. Eschlböck S, Delazer M, Krismer F, Bodner T, Fanciulli A, Heim B, et al. Cognition in multiple system atrophy: a single-center cohort study. Ann Clin Transl Neurol 2020;7:219–228.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite

