Articles
- Page Path
- HOME > J Mov Disord > Volume 17(1); 2024 > Article
-
Original Article
Hair Loss: A Well-Known Yet Understudied Symptom in Parkinson’s Disease Patients During Dopaminergic Therapy -
Jungyeun Lee1
 , Hwa Jung Ryu2
, Hwa Jung Ryu2 , Soon Young Hwang3
, Soon Young Hwang3 , Seong-Beom Koh1
, Seong-Beom Koh1

-
Journal of Movement Disorders 2024;17(1):47-54.
DOI: https://doi.org/10.14802/jmd.23088
Published online: September 26, 2023
1Department of Neurology and Parkinson’s Disease Center, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
2Department of Dermatology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
3Department of Biostatistics, Korea University College of Medicine, Seoul, Korea
- Corresponding author: Seong-Beom Koh, MD, PhD Department of Neurology and Parkinson’s Disease Center, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 08308, Korea / Tel: +82-2-2626-3169 / Fax: +82-8-2626-2249 / E-mail: parkinson@korea.ac.kr
Copyright © 2024 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,055 Views
- 84 Download
ABSTRACT
-
Objective
- Hair loss has been reported to occur during dopaminergic therapy in patients with Parkinson’s disease. The mechanism by which dopaminergic therapy induces hair loss is not well understood. Dopamine receptors are present in the hair follicle, where they regulate melanin production. However, the role of dopamine receptors in hair growth is still not well understood. This study aimed to evaluate the prevalence of hair loss and identify factors associated with complaints of hair loss in patients with Parkinson’s disease.
-
Methods
- A cross-sectional design involving 495 Parkinson’s disease patients was applied to evaluate hair loss status. Patients completed a questionnaire, and scalp/hair examinations were performed. Patients with underlying conditions that could affect hair loss and those prescribed medications known to increase the risk of hair loss were excluded. Finally, 291 patients (58.8%) were included for analysis.
-
Results
- Among the 495 patients, 138 (27.9%) reported hair loss. Interestingly, more than half of the patients who complained of hair loss (79 out of 138) did not utilize treatments such as hair products, massage, dietary modifications, or alopecia medications. Hair inspection by a single investigator revealed objective hair loss in 263 patients (53.1%). An analysis of factors associated with hair loss complaints showed that the intake of dopaminergic medications with a levodopa-equivalent daily dose > 448 mg was associated with complaints of hair loss.
-
Conclusion
- Dopaminergic medication is associated with hair loss complaints in Parkinson’s disease patients.
- The discovery of levodopa in 1960 [1] was followed by its introduction in pharmacological therapies for Parkinson’s disease (PD) to enhance synaptic dopamine transmission [2]. Currently, dopamine-based medications, including levodopa preparations and dopamine agonists, are widely used to alleviate PD symptoms [3,4].
- Patients taking dopaminergic medications sometimes complain of hair loss. Marshall et al. [5] first reported hair loss as a side effect of levodopa administration in 1971. Levodopa, as well as ergot alkaloid and non-ergot alkaloid dopamine agonists, can cause hair loss. Blum et al. [6] and Fabre et al. [7] reported cases of hair loss induced by bromocriptine administration, and Tabamo et al. [8] presented two cases of hair loss caused by pramipexole and ropinirole. Grauer et al. [9] and Factor et al. [10] reported hair loss as a side effect of cabergoline and pergolide, respectively. The mechanism by which dopaminergic treatment causes hair loss is not fully understood. Although several case reports of hair loss in PD patients have been published, the epidemiology and risk factors remain poorly understood, as limited data are available.
- In this study, the status of the hair and scalp in PD patients was investigated, and the association between the use of dopaminergic medications and hair loss in PD patients was assessed by administering a survey on scalp conditions and performing examinations.
INTRODUCTION
- Patients
- This was a cross-sectional observational study conducted at the Korea University Guro Hospital, a tertiary care center in Seoul, Korea, from August 2021 to February 2022. A total of 495 patients diagnosed with PD were included in the descriptive study to investigate hair/scalp status. The inclusion criteria were as follows: a diagnosis of PD by an experienced movement disorder specialist according to the United Kingdom Parkinson’s Disease Society Brain Bank criteria [11] and sufficient cognitive ability to complete the questionnaires. Patients diagnosed with multiple system atrophy, progressive supranuclear palsy, or vascular parkinsonism were excluded.
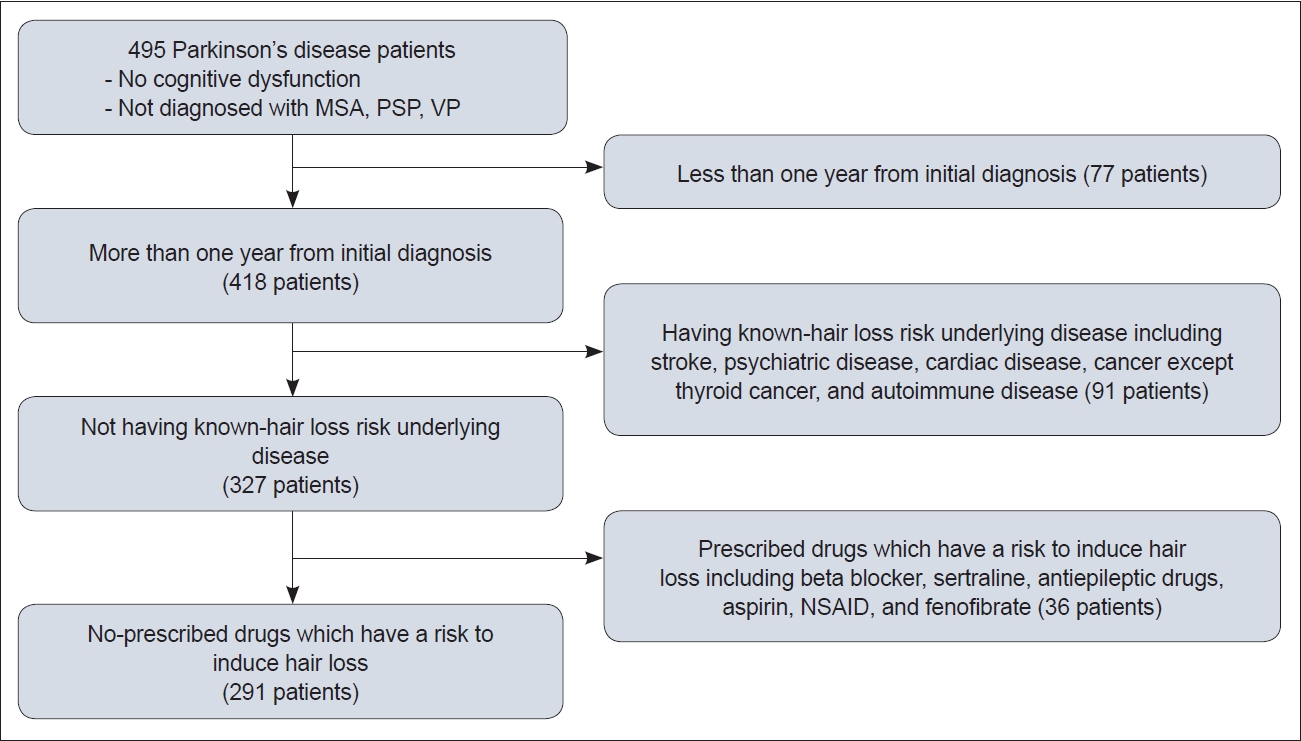
- To analyze the factors associated with hair loss in PD, patients meeting the following criteria were excluded: treatment for less than one year, the presence of underlying diseases known to have a risk of inducing hair loss, or the presence of diseases requiring hair loss-inducing medications as treatment (such as stroke, cardiac diseases, psychiatric diseases, autoimmune disorders, and cancers other than thyroid cancer). It should be noted that because the majority of the patients were elderly individuals, their ability to recall their underlying diseases and medication history accurately may have been limited. Considering this limitation, criteria were established to exclude patients based not only on medication history but also on medical conditions. Additionally, patients who were prescribed medications that can potentially cause hair loss (including beta-blockers, sertraline, carbamazepine, aspirin, fenofibrates and nonsteroidal anti-inflammatory drugs) were also excluded. Finally, a total of 291 patients were included in the analysis (Figure 1).
- Ethical approval was obtained from the Institutional Review Board of the Korea University Guro Hospital (IRB number: 2021GR0381). Informed consent was obtained from the patients before inclusion in the study.
- Data collection
- Data were collected through a combination of methods, including reviewing electronic medical records, conducting surveys, and performing examinations of the patients’ hair and scalp. A single neurologist was responsible for collecting the data from patients who visited the outpatient clinic. The questionnaires used in the study included information on the patients’ past medical history, current medication, subjective hair loss (location), social history, nutritional status, dermatologic history (self-assessment of scalp and hair condition and lifestyle factors such as hairstyles involving hair permanents, hair coloring, hairdryer use or ponytails), family history of hair loss, and psychiatric factors (personality and sleep duration). The questionnaire, written in Korean, was developed in consultation with a dermatologist. The survey was carried out by the investigator through face-to-face interviews.
- Hair examination
- Scalp visual inspection and hair pull test were performed by a single investigator. During the scalp visual inspection, scalp color, presence of dandruff, pattern of alopecia [12], and hair loss scale were assessed using the Hamilton-Norwood classification for males and the Ludwig classification for females [13-15]. The hair pull test was conducted on all patients, where the investigator pulled 50 to 60 hairs using three fingers (thumb, index finger, and long finger) from the vertex, two parietal areas, and occipital area [16,17]. A positive result was defined as the extraction of more than 10% of hairs (5 to 6 hairs) from a specific scalp area in each trial [16]. If more than one scalp area showed a positive result, telogen or anagen effluvium were considered [16]. In addition, the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, which is a scale for patients undergoing chemotherapy, was applied [18]. According to the CTCAE, hair loss of less than 50% of normal is categorized as grade 1, and hair loss of 50% or more of normal is categorized as grade 2. For patients classified as having grade 2 hair loss, a wig or hair piece may be necessary if the patient wishes to completely conceal the hair loss.
- Factors associated with hair loss in PD
- A total of 291 patients were included for the analysis of factors associated with hair loss in PD. The patients were divided into two groups based on their response to the question “Do you think your hair loss occurred after taking PD medications?” The risk factors associated with hair loss were assessed in patients who answered “yes.” If patients reported hair loss but attributed it to a cause other than PD medication, the investigator marked their response as ‘no’ accordingly. The optimal threshold for the levodopa equivalent daily dose (LEDD) was based on Youden’s index, which maximizes the sum of sensitivity and specificity.
- Statistical analysis
- Baseline characteristics are presented as medians with ranges for continuous variables and as numbers with percentages for categorical variables. To analyze the associations between each potential risk factor and hair loss complaints in PD patients, a stepwise multiple regression analysis was conducted. Variables with p values < 0.25 in the univariable analysis were included in the multivariable logistic regression analysis. All statistical analyses were performed using IBM SPSS Statistics for Windows version 26.0 (IBM Corp., Armonk, NY, USA). P values were two-sided, and p < 0.05 was considered statistically significant.
MATERIALS & METHODS
- Status of hair loss in PD patients
- To provide statistical data on the hair and scalp conditions of PD patients in Korea, we conducted a descriptive study involving 495 patients. Supplementary Table 1 in the online-only Data Supplement presents the baseline characteristics of the patients. The median age was 69 years (range: 33–90 years), and 225 patients (45.5%) were male. Based on body mass index, 262 patients (52.9%) were overweight or obese. The median duration between PD diagnosis and the investigation was 54 months (range: 1–283 months), and the median duration between the onset of PD symptoms and the investigation was 68 months (range: 3–295 months).
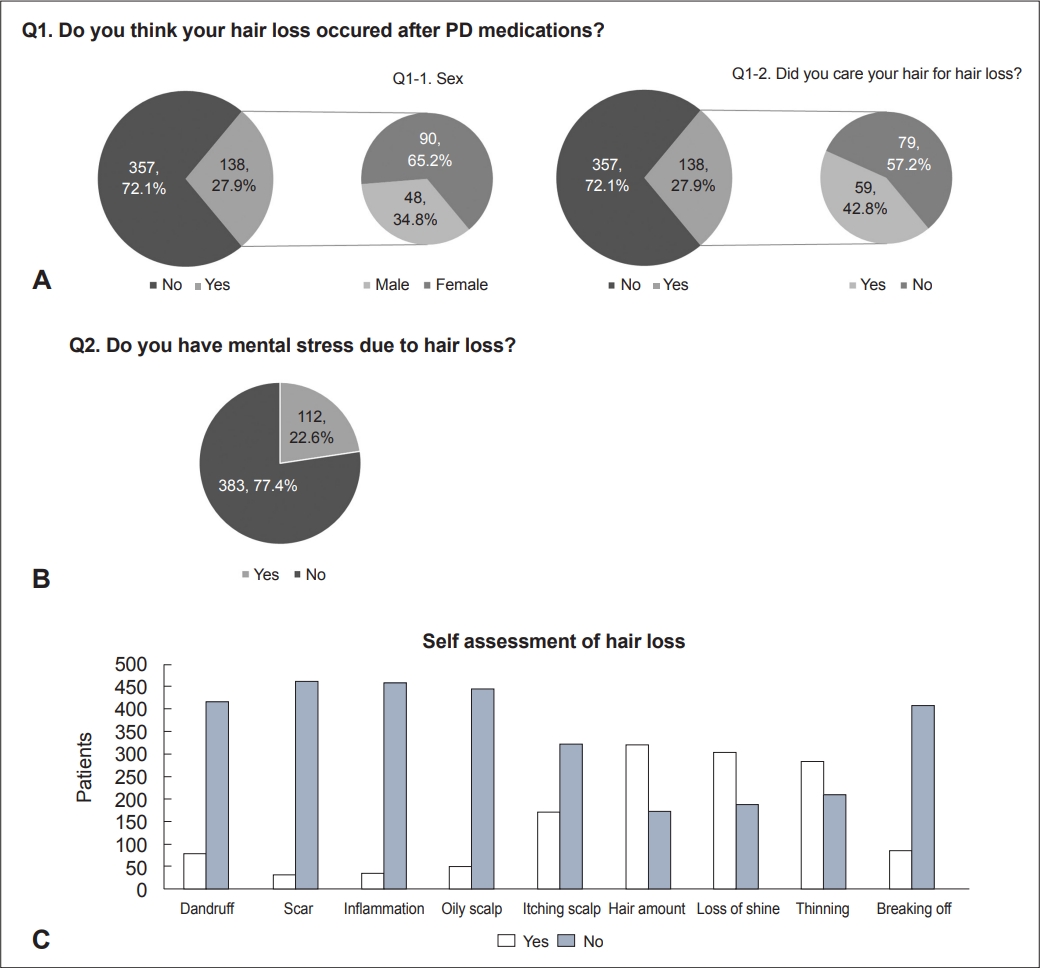
- Among a total of 495 PD patients, 138 (27.9%) and 112 (22.6%) provided a positive answer to the questions “Do you think your hair loss occurred after taking PD medications?” (Complaint of hair loss) and “Do you have mental stress due to hair loss?” (Distress from hair loss), respectively (Figure 2). Among the patients who complained of hair loss after taking PD medications, females (90 patients) reported hair loss more frequently than males (48 patients; 65.2% vs. 34.8%, p = 0.003). More than half of the patients who complained of hair loss (57.2%, 79 out of 138) did not use any treatments for hair loss, such as hair products, massage, dietary modifications, or alopecia medications. Figure 2 illustrates the self-assessment of hair and scalp status in PD patients. Most patients did not report dandruff, scars, inflammation, oily scalp, itching scalp, or hair breakage. Supplementary Table 1 in the online-only Data Supplement also shows the epidemiological data, including family history and history associated with hair loss. Among the patients, 332 patients (67.1%) reported no family members with hair loss, and 374 (75.6%) did not use any treatment for hair loss. The median numbers of hair permanents and hair colorings per year were 0 (range: 0–12) and 2.5 (range: 0–72), respectively. Twenty-six patients (5.3%) wore their hair in a ponytail style. Supplementary Table 1 in the online-only Data Supplement also provides information on the medication status of the patients. Most patients were prescribed levodopa (419, 84.6%). Dopamine agonists were prescribed to 242 (48.9%) patients. Among the dopamine agonists, pramipexole (209 patients) was the most prescribed, followed by ropinirole (32 patients) and bromocriptine (1 patient). Figure 3 and Supplementary Table 2 (in the online-only Data Supplement) show the results of hair and scalp examinations conducted by a single investigator. On scalp inspection, 263 patients (53.1%) showed signs of hair loss. Hair loss was categorized based on the distribution of loss on the scalp: patterned (143), diffuse (118) and focal (2). Based on the hair pull test (performed for all but four individuals who could not be evaluated due to short hair length), 450 patients (90.9%) had negative results. According to the CTCAE grade, hair loss was not suspected in 377 patients (76.2%, grade 0), and 118 patients (23.8%) were classified as having grade 1 or 2 hair loss. Figure 3 also shows the Norwood/Hamilton scale results for males and the Ludwig scale results for females.
- Risk factors associated with hair loss complaints in PD patients
- A total of 291 patients were enrolled for the analysis of risk factors associated with hair loss (Figure 1). Table 1 shows the baseline characteristics of these 291 patients. Table 2 presents the stepwise multiple regression analysis results for risk factors related to hair loss complaints. An LEDD > 448 mg and a high stress level were found to be associated with hair loss complaints in PD patients. However, sex, age, ponytail hairstyle, number of hair colorings, and history of dyslipidemia were not found to be associated with complaints of hair loss.
RESULTS
- Levodopa and dopamine agonists are the most frequently used drugs for PD. Hair loss induced by dopaminergic medications has sometimes been observed in PD patients [5-10]. In this study, the status of the hair and scalp was investigated, and the association between the use of dopaminergic medications and complaints of hair loss in PD patients was analyzed by administering a survey and performing scalp examinations.
- In the descriptive study that included 495 PD patients, 27.9% (138 out of 495) complained of hair loss due to medication, and more than half (57.2%, 79 out of 138) were not concerned about their hair loss. This lack of concern may be attributed to patients considering their hair loss inevitable and caused by other factors, such as aging and genetic factors. Additionally, more females complained of hair loss than males, which is consistent with findings from previous studies [5-8,19].
- The percentage of patients who complained of hair loss (27.9%) was greater than the objective hair loss observed in the hair pull test (8.3%). The hair pull test is a reliable method for assessing the ongoing activity and severity of any type of hair loss [20]. Positive results strongly indicate telogen effluvium, anagen effluvium, and early cases of patterned alopecia or an advancing edge of alopecia areata. However, negative hair pull test results do not exclude a hair loss diagnosis [21], and false-negative results are possible.
- To analyze factors associated with hair loss in PD patients, 204 out of 495 patients were excluded because they had other underlying diseases or were taking medications that can induce hair loss. Due to the advanced age of most patients, it was not possible to obtain accurate information on their past medication history. Therefore, individuals diagnosed with diseases requiring the use of high-risk drugs were excluded. Among the 291 patients included in the analysis, an association between objective hair loss based on the hair pull test results and dopaminergic medication use could not be statistically determined due to the small number of patients with positive hair pull test results (n = 11). However, an association between the LEDD and complaints of hair loss was observed. The LEDD cutoff point was determined at the point that maximizes the sum of sensitivity (0.837) and specificity (0.287). Although the specificity is low, the high sensitivity suggests the potential influence of dopaminergic drugs on increasing the risk of hair loss complaints. The risk of hair loss was 2.671 times higher in patients taking an LEDD > 448 mg based on multivariable analysis (p = 0.005).
- In previous studies, sex was suggested to affect dopaminergic medication-induced hair loss [19]. An in vitro study [22] reported that dopamine promotes hair follicle regression (catagen) in female anagen hair follicles. Dopamine is also associated with reduced proliferation of hair follicle matrix keratinocytes [22]. Because D2 receptors are located in the basal layer of the epidermis, D2-receptor agonist treatment can reduce epidermal hyperplasia and result in barrier disruption [22]. Additionally, females tend to be more sensitive and concerned regarding their appearance than males; thus, females may be more likely to answer “yes” to hair loss questions. In this study, the percentage of females complaining of hair loss was higher than that of males based on the descriptive test, which was consistent with previous studies [5-7,19]. Females showed a higher likelihood of hair loss in the multivariable analysis (adjusted odds ratio 1.549), but the difference was not statistically significant (p = 0.136).
- In this study, except for diffuse hair loss, females complained of hair loss in the frontotemporal and parietal areas more than males. This finding indicates a similarity with the dichotomous action of prolactin based on sex [23-25]. Prolactin can play various roles in hair follicles depending on sex. In the hair follicles of the frontotemporal scalp in females, prolactin downregulates the gene expression associated with follicular catagen. On the other hand, in hair follicles of the occipital scalp in males, prolactin induces hair follicle regression (catagen). While dopamine is generally known to inhibit prolactin, its inhibiting role in the hair follicle is not clearly understood, and tissue-specific effects may be present [26]. Therefore, it is difficult to attribute the results of the descriptive study solely to the influence of dopamine. This is because the potential impact of other hormones, such as prolactin and androgen, cannot be excluded. We were unable to obtain blood levels of prolactin and androgen for all patients, which can be considered a limitation of this study.
- To investigate the psychological impact of subjective hair oss complaints, we included personality-related questions in the survey. Contrary to the expectation that individuals with high levels of anxiety or sensitivity would be more affected, it was found that high stress levels were associated with hair loss complaints. In fact, high stress levels are known risk factors for common hair loss conditions such as androgenic alopecia, alopecia areata, and telogen effluvium [27-29]. In this study, patients with high stress levels showed a higher likelihood of in the univariable analysis, with an odds ratio of 1.796 (p = 0.024). In the multivariable analysis, the adjusted odds ratio increased to 2.671 (p = 0.005).
- Although aging is a well-known risk factor for hair loss [30,31], age was not significantly associated with hair loss in this study. Elderly patients tend to be less sensitive and concerned about their appearance than younger patients; they might consider their hair loss to be due to aging rather than PD medications.
- While several case reports have been published [5-10,19], to the best of our knowledge, this is the first study to investigate hair loss in PD patients and assess the association between dopaminergic medication use and complaints of hair loss. The mechanism of how dopamine affects hair loss is not yet fully understood; however, dopamine and dopamine agonists can impact human hair follicles through dopamine receptors [19,22] or prolactin.
- This study has several limitations. First, it was a single-center, observational study, which limits the generalizability of the findings. Second, the study results primarily relied on responses to a questionnaire survey conducted at a specific time, which introduces the potential for recall bias. Additionally, it was not feasible to determine the progression of hair loss based on the duration of medication use. Third, objective assessments such as blood tests, trichograms and trichoscopy were not available. Therefore, the diagnosis of specific hair loss conditions, such as androgenic alopecia, telogen effluvium, alopecia areata and others, was not possible. We acknowledge that the absence of significant results in the analysis of hair and scalp examination findings can be attributed to these limitations. However, even in the presence of these limitations, statistical data regarding subjective hair loss complaints can still provide valuable clinical insights.
- In summary, the intake of dopaminergic medication with an LEDD > 448 mg was associated with complaints of hair loss. Dopaminergic medication use may be associated with complaints of hair loss. These findings will be helpful to neurologists in PD clinics.
DISCUSSION
Supplementary Material
Supplementary Table 1.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None
-
Author contributions
Conceptualization: Seong-Beom Koh. Data curation: Jungyeun Lee. Formal analysis: Soon Young Hwang, Jungyeun Lee. Investigation: Jungyeun Lee. Methodology: Jungyeun Lee. Supervision: Hwa Jung Ryu, Seong-Beom Koh. Writing—original draft: Jungyeun Lee. Writing—review & editing: Seong-Beom Koh.
Notes

| Total (n = 291) | Complaint of hair loss (+) (n = 86) | Complaint of hair loss (−) (n = 205) | |
|---|---|---|---|
| Sex | |||
| Male | 142 (48.8) | 36 (41.9) | 106 (51.7) |
| Female | 149 (51.2) | 50 (58.1) | 99 (48.3) |
| Age, yr* | 68.23 (33–89) | 67.10 (41–89) | 68.70 (33–89) |
| BMI* | 23.39 (8.86–34.75) | 23.18 (8.86–29.90) | 23.48 (15.76–34.75) |
| Sufficient nutrition (history) | 263 (90.4) | 76 (88.4) | 187 (91.2) |
| Weight loss (history) | 43 (14.8) | 14 (16.3) | 29 (14.1) |
| Family history of hair loss | 101 (34.7) | 32 (37.2) | 69 (33.7) |
| Ponytail hairstyle | 19 (6.5) | 9 (10.5) | 10 (4.9) |
| Perming count (per year)* | 1.54 (0–12) | 1.45 (0–6) | 1.58 (0–12) |
| Dyeing count (per year)* | 5.13 (0–72) | 6.12 (0–24) | 4.71 (0–72) |
| Dryer use (per week)* | 1.32 (0–7) | 1.41 (0–7) | 1.28 (0–7) |
| Hypertension | 107 (36.8) | 29 (33.7) | 78 (38.0) |
| Diabetes | 45 (15.5) | 13 (15.1) | 32 (15.6) |
| Dyslipidemia | 26 (8.9) | 5 (5.8) | 21 (10.2) |
| Thyroid | 15 (5.2) | 4 (4.7) | 11 (5.4) |
| Dermatologic disease | 21 (7.2) | 8 (9.3) | 13 (6.3) |
| Sensitive personality (questionnaire) | 151 (51.9) | 52 (60.5) | 99 (48.3) |
| Anxiety (questionnaire) | 156 (53.6) | 48 (55.8) | 108 (52.7) |
| Tendency towards easily getting angry (questionnaire) | 76 (26.1) | 25 (29.1) | 51 (24.9) |
| High stress level (questionnaire) | 136 (46.7) | 49 (57.0) | 87 (42.4) |
| Drug levodopa use | 255 (87.6) | 76 (88.4) | 179 (87.3) |
| Drug dopamine agonist use | 163 (56.0) | 49 (57.0) | 114 (55.6) |
| Levodopa-equivalent daily doses† | 600 (440–800) | 606 (450–856) | 575 (400–799) |
| Symptom-to-test, month* | 16.20 (0–123) | 15.96 (0–120) | 16.30 (0–123) |
| Diagnosis-to-test, month* | 76.04 (13–283) | 71.56 (15–230) | 77.93 (13–283) |
| Variables |
Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | AOR | 95% CI | p value | |
| Sex, female | 1.487 | 0.894–2.473 | 0.126 | 1.549 | 0.872–2.752 | 0.136 |
| Age | 0.985 | 0.961–1.009 | 0.224 | 0.978 | 0.951–1.004 | 0.102 |
| BMI > 23 | 1.021 | 0.615–1.692 | 0.937 | |||
| Nutrition (history) | 1.367 | 0.603–3.097 | 0.454 | |||
| Weight loss (history) | 1.180 | 0.589–2.363 | 0.640 | |||
| Family history of hair loss | 1.168 | 0.691–1.973 | 0.562 | |||
| Ponytail hairstyle | 2.279 | 0.892–5.825 | 0.085 | 2.095 | 0.731–6.007 | 0.169 |
| Perming count (per year) | 0.977 | 0.876–1.090 | 0.679 | |||
| Dyeing count (per year) | 1.021 | 0.990–1.053 | 0.181 | 1.022 | 0.990–1.056 | 0.183 |
| Dryer use (per week) | 1.025 | 0.920–1.143 | 0.652 | |||
| Hypertension | 0.828 | 0.488–1.405 | 0.485 | |||
| Diabetes | 0.963 | 0.478–1.939 | 0.915 | |||
| Dyslipidemia | 0.541 | 0.197–1.485 | 0.233 | 0.394 | 0.136–1.145 | 0.087 |
| Thyroid | 0.860 | 0.266–2.781 | 0.802 | |||
| Dermatologic disease | 1.515 | 0.604–3.798 | 0.376 | |||
| Sensitive personality (questionnaire) | 1.638 | 0.982–2.732 | 0.059 | 1.387 | 0.770–2.497 | 0.275 |
| Anxiety (questionnaire) | 1.135 | 0.684–1.882 | 0.625 | |||
| Tendency towards easily getting angry (questionnaire) | 1.238 | 0.705–2.173 | 0.458 | |||
| High stress level (questionnaire) | 1.796 | 1.080–2.988 | 0.024* | 1.992 | 1.117–3.553 | 0.020* |
| Drug levodopa | 1.104 | 0.508–2.401 | 0.803 | |||
| Drug dopamine agonist | 1.057 | 0.636–1.757 | 0.830 | |||
| Levodopa-equivalent daily doses > 448 mg | 2.078 | 1.088–3.971 | 0.027* | 2.671 | 1.340–5.325 | 0.005* |
| Symptom-to-test (months) | 0.998 | 0.994–1.002 | 0.281 | |||
| Diagnosis-to-test (months) | 0.998 | 0.993–1.003 | 0.351 |
- 1. Birkmayer W, Hornykiewicz O. [The L-3,4-dioxyphenylalanine (DOPA)- effect in Parkinson-akinesia]. Wien Klin Wochenschr 1961;73:787–788.German. PubMed
- 2. Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 2015;132:96–168.ArticlePubMed
- 3. Mercuri NB, Bernardi G. The ‘magic’ of L-dopa: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol Sci 2005;26:341–344.PubMed
- 4. Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA 2020;323:548–560.ArticlePubMed
- 5. Marshall A, Williams MJ. Alopecia and levodopa. Br Med J 1971;2:47.ArticlePubMedPMC
- 6. Blum I, Leiba S. Increased hair loss as a side effect of bromocriptine treatment. N Engl J Med 1980;303:1418.Article
- 7. Fabre N, Montastruc JL, Rascol O. Alopecia: an adverse effect of bromocriptine. Clin Neuropharmacol 1993;16:266–268.PubMed
- 8. Tabamo RE, Di Rocco A. Alopecia induced by dopamine agonists. Neurology 2002;58:829–830.ArticlePubMed
- 9. Grauer MT, Sieb JP. Alopecia induced by dopamine agonists. Neurology 2002;59:2012.Article
- 10. Factor SA, Sanchez-Ramos JR, Weiner WJ. Parkinson’s disease: an open label trial of pergolide in patients failing bromocriptine therapy. J Neurol Neurosurg Psychiatry 1988;51:529–533.ArticlePubMedPMC
- 11. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184.ArticlePubMedPMC
- 12. Mirmirani P, Fu J. Diagnosis and treatment of nonscarring hair loss in primary care in 2021. JAMA 2021;325:878–879.ArticlePubMed
- 13. Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 1977;97:247–254.ArticlePubMed
- 14. Norwood OT. Male pattern baldness: classification and incidence. South Med J 1975;68:1359–1365.ArticlePubMed
- 15. Blume-Peytavi U, Blumeyer A, Tosti A, Finner A, Marmol V, Trakatelli M, et al. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol 2011;164:5–15.ArticlePubMed
- 16. McDonald KA, Shelley AJ, Colantonio S, Beecker J. Hair pull test: evidence-based update and revision of guidelines. J Am Acad Dermatol 2017;76:472–477.ArticlePubMed
- 17. McDonald KA, Shelley AJ, Maliyar K, Abdalla T, Beach RA, Beecker J. Hair pull test: a clinical update for patients with Asian- and Afro-textured hair. J Am Acad Dermatol 2021;85:1599–1601.ArticlePubMed
- 18. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90–92.ArticlePubMed
- 19. Miwa H, Kondo T. Hair loss induced by dopamine agonist: case report and review of the literature. Parkinsonism Relat Disord 2003;10:51–52.ArticlePubMed
- 20. Van Neste MD. Assessment of hair loss: clinical relevance of hair growth evaluation methods. Clin Exp Dermatol 2002;27:358–365.ArticlePubMedPDF
- 21. Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology 2009;1:108–119.ArticlePubMedPMC
- 22. Langan EA, Lisztes E, Bíró T, Funk W, Kloepper JE, Griffiths CE, et al. Dopamine is a novel, direct inducer of catagen in human scalp hair follicles in vitro. Br J Dermatol 2013;168:520–525.ArticlePubMed
- 23. Paus R, Langan EA, Vidali S, Ramot Y, Andersen B. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med 2014;20:559–570.ArticlePubMed
- 24. Langan EA, Ramot Y, Goffin V, Griffiths CE, Foitzik K, Paus R. Mind the (gender) gap: does prolactin exert gender and/or site-specific effects on the human hair follicle? J Invest Dermatol 2010;130:886–891.ArticlePubMed
- 25. Langan EA, Griffiths CE, Paus R. Utilizing the hair follicle to dissect the regulation and autocrine/paracrine activities of prolactin in humans. Am J Physiol Endocrinol Metab 2012;302:E1311–E1312.ArticlePubMed
- 26. Grymowicz M, Rudnicka E, Podfigurna A, Napierala P, Smolarczyk R, Smolarczyk K, et al. Hormonal effects on hair follicles. Int J Mol Sci 2020;21:5342.ArticlePubMedPMC
- 27. Gupta MA, Gupta AK, Watteel GN. Stress and alopecia areata: a psychodermatologic study. Acta Derm Venereol 1997;77:296–298.ArticlePubMedPDF
- 28. Cash TF. The psychology of hair loss and its implications for patient care. Clin Dermatol 2001;19:161–166.ArticlePubMed
- 29. Chien Yin GO, Siong-See JL, Wang ECE. Telogen effluvium - a review of the science and current obstacles. J Dermatol Sci 2021;101:156–163.ArticlePubMed
- 30. Park AM, Khan S, Rawnsley J. Hair biology: growth and pigmentation. Facial Plast Surg Clin North Am 2018;26:415–424.PubMed
- 31. Trüeb RM, Rezende HD, Dias MFRG. A comment on the science of hair aging. Int J Trichology 2018;10:245–254.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite