Articles
- Page Path
- HOME > J Mov Disord > Volume 14(1); 2021 > Article
-
Original Article
The Queensland Parkinson’s Project: An Overview of 20 Years of Mortality from Parkinson’s Disease -
Peter Cornelis Poortvliet1
 , Alexander Gluch1, Peter A. Silburn2
, Alexander Gluch1, Peter A. Silburn2 , George D. Mellick1
, George D. Mellick1
-
Journal of Movement Disorders 2021;14(1):34-41.
DOI: https://doi.org/10.14802/jmd.20034
Published online: December 7, 2020
1Griffith Institute for Drug Discovery, School of Environment and Science, Griffith University, Brisbane, Australia
2Queensland Brain Institute, University of Queensland, Brisbane, Australia
- Corresponding author: Peter Cornelis Poortvliet, PhD Griffith Institute for Drug Discovery, Griffith University, Building N27, Nathan Campus, 4011 QLD, Australia / Tel: +61-7-3735-6015 / E-mail: p.poortvliet@griffith.edu.au
Copyright © 2021 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- The consensus is that life expectancy for individuals with Parkinson’s disease (PD) is reduced, but estimations vary. We aimed to provide an overview of 20 years of mortality and risk factor data from the Queensland Parkinson’s Project.
-
Methods
- The analysis included 1,334 PD and 1,127 control participants. Preliminary analysis of baseline characteristics (sex, age at onset, family history, smoking status, pesticide exposure, depression and neurosurgery) was conducted, and Kaplan–Meier curves were generated for each potential risk factor. Standardized mortality ratios (SMRs) were calculated comparing this cohort to the general Australian population. Cox proportional hazards regression modeling was used to analyze potential predictors of mortality.
-
Results
- In total, 625 (46.8%) PD and 237 (21.0%) control participants were deceased. Mean disease duration until death was 15.3 ± 7.84 years. Average ages at death were 78.0 ± 7.4 years and 80.4 ± 8.4 years for the deceased PD and control participants, respectively. Mortality was significantly increased for PD in general {SMR = 2.75 [95% confidence interval (CI): 2.53–2.96]; p = 0.001}. SMRs were slightly higher for women and those with an age of onset before 60 years. Multivariate analysis showed that deep brain stimulation (DBS) treatment was associated with lower mortality [hazard ratio (HR) = 0.76; 95% CI: 0.59–0.98], while occasional pesticide exposure increased mortality risk (HR = 1.48; 95% CI: 1.17–1.88). Family history of PD, smoking and depression were not independent predictors of mortality.
-
Conclusion
- Mortality in PD is increased. Sex, age at onset and occasional pesticide exposure were independent determinants of increased mortality, while DBS treatment was associated with reduced mortality.
- Participants
- The data for analyses were obtained from the QPP, a longitudinal community-based research collaborative, initiated in 2000, aimed at identifying risk and progression markers for PD. QPP participation information is widely disseminated among movement disorder clinics in Queensland. Additionally, project information can be found online on the university website as well as the website of PD support groups. Volunteers express their interest in participation, after which they receive a participation pack including the project information and consent documentation. A control group, comprising of relatives, friends or individuals identified from the commonwealth electoral roll, was used for analysis. Baseline personal and medical information and a blood sample are collected upon entry, and any changes in personal and medical circumstances are collected through annual standardized follow up. The QPP has been approved by local ethics (IRB 2011-730), and all the participants signed informed consent upon entry to the QPP. Different methods were used to monitor vital status, including annual follow-ups and national death registries. Entries for which the vitality status could not be obtained were excluded from analysis. By the census date (February 2020), 1,334 participants diagnosed with PD were included in this study as well as 1,127 controls for comparison, where appropriate.
- Statistical analysis
- Standardized mortality ratios (SMRs) were calculated for the PD cohort at the census date for men and women using 5-year age groups. Additionally, SMRs were calculated separately for those with a disease onset age before 60 years and 60 years or older. SMRs were considered significant if the lower limit of the 95% confidence interval (CI) was higher than 1 or if the upper limit was below 1. The expected number of deaths was calculated using the age- and sex-specific population data from the Australian Bureau of Statistics, with a 5-year age interval [9].
- Survival was also calculated in years from the age of PD onset to age at death or censoring. A Kaplan–Meier plot was generated for survival analysis. The log-rank test was used in univariate analyses to evaluate differences between the deceased and survival groups. To investigate potential independent risk factors, a Cox proportional hazards model was applied based on multivariate analysis using the following categorical predictors: age at onset (year of the first symptom), sex, pesticide exposure [never, occasional (< 24 days), regular (> 25 days)], family history of PD, smoking status (ever vs. never), neurosurgery [deep brain stimulation (DBS) and pallidotomy] and depression (Geriatric Depression Scale score > 6 or previous treatment for depression). Continuous variables were expressed as the means ± standard deviation (SD). All information was collected using a structured questionnaire [10]. Values of p < 0.05 were considered statistically significant.
METHODS
- An overview of the baseline characteristics of the study population is found in Table 1. During the 20-year period, the average follow-up duration for the PD cohort was 8.1 ± 4.2 years (10,711 person-years available for analysis) and 10.1 ± 3.76 years (11,364 person-years) for the control group. Most of the included PD participants were male (845/1,334; 63.3%). The average age of PD onset was 59.5 ± 11.4 years, which was similar for both men and women (Table 1), and the average age at death or census was 78.0 ± 7.4 years. The sex distribution of the control group was more evenly matched (546/1,127; 48.5% male). The disease duration for the PD cohort was 16.5 ± 7.7 years, with marginally longer durations for women than men, 17.1 ± 8.0 and 16.2 ± 7.6 years, respectively. A family history of PD was reported by 28.4% and 34.2%, depression by 41% and 18.3%, smoking history by 50.2% and 55.8% and neurosurgery by 19.6% and 0% of PD and control participants, respectively. Most of the participants had never been exposed to pesticides (71.1% and 82.3%, PD and control participants, respectively). Most of those with occasional (9.1% vs. 6.0%) and regular (18.7% vs. 11.4%) exposure were male (68.0% vs. 61.8% and 82.7% vs. 81.4% for PD vs. controls, respectively).
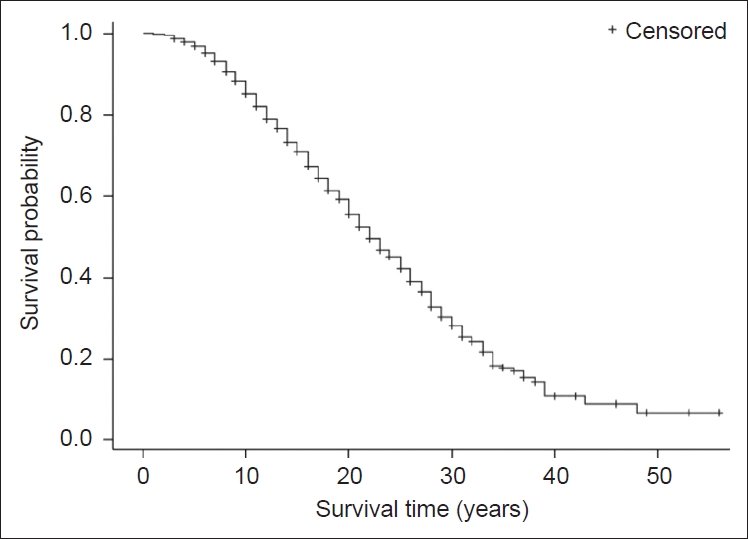
- Of the 1,334 PD participants included in the analyses, 625 (46.8%) were deceased, with an average follow-up duration of 5.5 ± 3.3 years. The average age at onset of the deceased in the PD group was 62.7 ± 10.6 years, and the average age at death was 78.0 ± 7.4 years, whereas the average age of onset for survivors in the PD group was 56.6 ± 11.3 years and age at census was 74.1 ± 9.2 years. The average follow-up duration for the survivors in the PD group was 10.3 ± 3.6 years. Initial exploration of the data using Kaplan–Meier survival analysis (Figure 1) indicated a median time of survival from the age of onset of 23.8 years with a range of 1–56 years for the entire PD cohort. The probability of survival was reduced to 0.83 after 10 years of disease duration, after which survival was significantly decreased (Figure 1).
- In the control group, 237 (21.0%) of the 1,127 participants had died, with an average follow-up duration of 5.4 ± 4.0 years. The average age at entry was 65.8 ± 10.9 years; the age at death for the deceased controls was 80.4 ± 8.4 years, and the age at census was 74.7 ± 10.7 years for the survivors.
- An overview of the SMRs for the PD cohort is shown in Table 2. Mortality was significantly increased in the PD cohort compared with that in the general Australian population as well as the population of the state of Queensland [SMR=2.75 (95% CI: 2.53–2.96) and 2.70 (95% CI: 2.49–2.91), respectively]. Compared with national and local expected deaths, women in the PD group showed a slightly higher mortality rate than men (Table 2). Mortality was increased regardless of the age of onset; however, mortality was higher in the group with a disease onset before the age of 60 years.
- Table 3 presents the results of the Cox hazard modeling for the PD group. Initial univariate development of the model revealed later age at onset (p = 0.001) and male sex (p = 0.001) as strong independent predictors of decreased survival. After adjusting for age and sex, DBS surgery (p = 0.012) was associated with increased survival and occasional pesticide exposure (p = 0.001) showed a significant association with reduced survival. DBS treatment demonstrated a moderately lower risk of death [hazard ratio (HR) = 0.73], while occasional pesticide exposure showed a substantial increased risk in mortality (HR = 1.52). Regular pesticide exposure showed a moderately increased mortality risk (HR = 1.21); however, this finding was not statistically significant (p = 0.074). The results of multivariate modeling indicated similar findings. Male sex (HR = 1.31), age at onset (HR = 1.11) and occasional pesticide exposure (HR = 1.48) all showed a statistically significant increase in mortality (p < 0.01). Additionally, DBS treatment was associated with increased survival (HR = 0.76) that was statistically significant (p = 0.038). Other baseline variables were not independent predictors of mortality in both univariate and multivariate analyses.
- Table 4 presents the results of Cox hazard modeling for the control group. Both univariate and multivariate analyses showed a significant impact of occasional pesticide exposure on mortality (HR = 2.58 and HR = 2.83, respectively, both p = 0.001). All other variables showed no significant effect.
RESULTS
- This study presents mortality estimations on various risk factors from the 20 years of systematic Australian population-based PD data collection in the QPP database. Consistent with findings from previous studies, our results showed increased mortality in PD. Furthermore, older age at onset, male sex and occasional pesticide exposure were associated with increased mortality, whereas previous DBS surgery was associated with decreased mortality.
- Mortality in this PD cohort was significantly increased compared with both the general Australian population and Queensland state population. This finding agrees with the data in previous reports, although our SMRs show higher ranges than those reported previously [2]. The common limitations of those studies are the short duration and small sample sizes, which could explain the lower mortality estimations [2]. Diem-Zangerl et al. [3] reported increased SMRs at lower ranges that increased over time in their 20-year follow-up study [SMR = 0.6 (95% CI: 0.4–1.0) by 5 years, 0.9 (95% CI: 0.7–1.2) by 10 years, 1.2 (95% CI: 1.0–1.4) by 15 years, and 1.3 (95% CI: 1.1–1.5) by 20 to 30 years]. The Sydney multicenter study also reported significant differences in SMR with increasing disease durations that were more in common with our findings. For example, their results showed increased SMRs from 0.5 after 3 years of disease, to 1.8 after 5 years, 2.3 between 5 and 10 years, 2.7 at 10–15 years and 3.1 at 15–20 years [4].
- The median probability of survival of our cohort was 23.78 years, which is considerable. In a recent comprehensive review by Macleod et al. [2], 88 mortality studies in PD were compared and the outcomes were analyzed. They found 18 studies that reported a median survival, which ranged between 6 and 22 years. The authors concluded that the major discrepancy between the reported survival estimates was due to differences in baselines (e.g., time of diagnosis, onset or recruitment). The estimations in the current study were based on the year of onset, which might explain the long survival. Generally, the diagnostic delay (from the onset of the first motor symptom to diagnosis) is estimated to be approximately 12–15 months [11,12], whereas recruitment can occur at any stage before the onset of or during the disease. Similar to the studies reviewed previously, the probability of survival remains relatively constant during the first few years and then declines steadily. The average decline in survival in the reviewed studies was approximately 5% per year compared with 2.5% in the current study.
- The average disease duration until death of 15.3 years in the current study was longer than that in previous reports. A metaanalysis performed by Macleod et al. [2] identified 10 studies (n = 1,306) that reported highly variable disease durations ranging from 6.9 to 14.9 years. In particular, a previous PD mortality study conducted in Australia found an average disease duration of 9.1 years [4].
- The average age at death for men was 77.6 ± 7.0 years and that for women was 78.8 ± 8.0 years, which were less than the average ages at death in the control group (80.4 ± 7.8 years and 80.4 ± 9.1 years for men and women, respectively). The estimated life expectancy of the general public in Queensland of 80.5 years for men and 85.0 years for women [13] agrees with that of the control group. Although some studies reported comparable PD mortality rates to that of the general population, the period of follow up of those studies was usually short and the cohorts were small. The findings from studies with a longer follow up, however, seem to suggest similar or moderately increased PD mortality compared with that of the general population for the first 10 years, after which the mortality rate increases steadily until 20 years and does not increase much further [4,6,14,15].
- Consistent with previous studies on mortality in PD, survival analysis of the potential risk factors in multivariate analysis indicated that age at onset and sex were independent predictors of mortality. The increased mortality risk from the age at onset (HR = 1.11) matches that in previous reports suggesting approximately 7–10% increased risk for each additional year after onset [3,4,6-8]. Similarly, the SMRs showed increased mortality in PD regardless of the age of onset. However, a younger age of onset showed a considerably higher mortality than an onset after the age of 60 years. These findings are consistent with those in previous studies suggesting an increased risk of mortality with a lower age of disease onset (before the age of 60 years) [3,5,6,16,17]. Except for Morgan et al. [16], those studies generally showed increased mortality in both groups. Others reported that older age, irrespective of onset, was an independent risk factor for increased mortality [7,14,18], an expected finding because increasing age is associated with increased mortality in the general population.
- Male sex was associated with moderately increased mortality compared with female sex (HR = 1.31). However, the SMRs of the current study showed the opposite, with slightly higher mortality for women regardless of the age of onset. This finding is in contrast to that in the Sydney multicenter study in which sexadjusted SMR values indicated equivocal mortality between men and women with PD [4]. Commonly, male sex is found to have a higher mortality risk. For example, a Norwegian study found that male sex was associated with an increased risk by 63% (HR = 1.63) [19] and other studies showed similar findings [3,6]. However, sex is not consistently found to be a statistically significant predictor of mortality [4,8,20]. The increase in mortality for men with PD is potentially due to the increased mortality experienced by the general male population. Our results could have been influenced by more men than women having received DBS surgery, which was associated with a reduced risk of mortality in our study.
- Interestingly, DBS treatment was associated with reduced mortality. Although DBS treatment has been suggested to have neuroprotective effects and studies have reported decreased mortality in individuals with PD treated with DBS, controversy persists regarding these claims. Kaplan–Meier curve analysis indicated a strong association of DBS treatment compared with the rest of the cohort. This finding was further supported by the Cox proportional hazards regression model, in which both uni- and multivariate analyses showed significant associations (p < 0.05). The HR of 0.76 (95% CI: 0.59–0.98) suggested mildly lower mortality rates for those with DBS treatment when adjusted for all other variables. These findings are similar to those in previous studies. Hitti et al. [21], for example, showed a survival of 51% after 10 years in a cohort of 200 participants with DBS treatment. Ngoga et al. [22] also found a significant effect of DBS treatment on mortality compared with a pharmacological treatment group (HR = 0.29; 95% CI: 0.13–0.64; p < 0.01). By contrast, Schüpbach et al. [23] found no difference in survival after secondary analysis comparing a DBS and a non-DBS group. Similarly, a study conducted at the Oslo University Hospital also found no effects of DBS treatment on mortality [24]. However, both studies matched and compared their DBS treatment cohort to nontreatment groups from different sources (different countries [23] and time periods [23,24]), making direct comparisons questionable. Several reasons could be proposed for the associations of DBS treatment with reduced PD mortality. First, the selection of DBS treatment often requires an excellent levodopa response, little comorbidity and no cognitive deficits, which could explain the longer survival. Patients who are deemed medically fit and expected to live longer are more often viable candidates for this treatment option. However, DBS treatment is commonly preferred when pharmacological treatment is no longer effective and high dosages cause several complicating side effects that significantly affect health and quality of life. The second reason for the DBS effects on mortality are that treatment often results in the reduction in the traditional medication regimen. Toft et al. [25] showed that levodopa was reduced by 49% following DBS surgery and remained lower throughout subsequent management. In the current study, we only compared pre- and post-DBS surgery medications for a subgroup of 73 patients (47 men). At baseline, the average levodopa equivalent dose was calculated as 762.27 ± 465.21 mg/day. Six months following DBS surgery, the average dose was 279.90 ± 274.35 mg/day, a significant average reduction of 63.39% (2-tailed t-test: p < 0.001). Importantly, our study makes no implications about the toxicity of PD medication. Instead, as pointed out by Ngoga et al. [22], several studies have reported on the beneficial effects of improved quality of life, motor function and reductions in drug-related side effects following surgery. Together, these effects are suggested to reduce the risk of respiratory disease. Indeed, in their study, more medically managed PD patients died of respiratory causes (pneumonia and pulmonary embolism) than those treated with DBS [22]. Another potential reason for the associations of DBS with mortality is the intensified disease management following DBS surgery that requires (and is a selection prerequisite) frequent medical follow-up [26]. Therefore, potential disease complications and comorbidities are more intensively screened and monitored and, where possible, treated, which could be more beneficial for overall health [27].
- Pesticide exposure at occasional levels (HR = 1.48; 95% CI: 1.17–1.88), but not regular levels (HR = 1.20; 95% CI: 0.97–1.49), showed a significantly increased risk of mortality. Interestingly, occasional pesticide exposure was also a significant predictor of mortality in the control group. Although the association between pesticide exposure and PD has been studied extensively, the relationship between exposure and mortality risk in PD has only received modest attention. The study by Ritz and Yu [28] was one of the first to compare PD mortality in counties in California, USA with high agricultural pesticide use with those not using pesticides. Their results showed a clear dose-response relationship for insecticide use per county land treated and risk of mortality [28]. A Dutch occupational hazard study on PD found that pesticide exposure was associated with an elevated risk of mortality among men, but among the ‘ever high exposed’ subgroup (HR = 1.27; 95% CI: 0.86–1.88) and not the ‘ever only low’ group [29]. Statistical significance was only revealed for the 1st tertile when cumulative exposures were considered [HR = 1.89 (95% CI: 1.11–3.22) for the group with the lowest unit years of exposure]. Interestingly, a previous study investigating mortality from PD and other causes among workers manufacturing the potent herbicide paraquat found no evidence of increased mortality from PD. All-cause mortality of the workers was significantly lower than that of the general population [30]. Of the 292 deaths over 10 years, only two workers had PD mentioned as the cause of death on their death certificate. Underlying PD is not often listed as the cause of death on death certificates [31] and could have resulted in an underestimation of the total PD deaths [30]. Our report, in addition to the previous Californian and Dutch study, seems to indicate that pesticide exposure may impact mortality and that further investigation into this topic is required.
- There are several limitations of this study. First, a limited number of risk factors were investigated with potential confounding variables not considered. This includes mental abnormalities, such as dementia and psychosis, which are known to impact mortality [4,7,20,32]. Other potential risk factors not investigated in this study include the impact of sleep or clinical phenotype. This information was not collected consistently enough for further analyses.
- Additionally, most variables were recorded at baseline with some of the recorded variables only based on approximate patient recall, which can result in recall bias impacting the accuracy of the data. Although having consistent follow-up and accurately measuring these variables would significantly improve the validity of the data, this is difficult for studies of this scale and time span.
- Likewise, there may be selection bias resulting in the misrepresentation of the total population with PD in Queensland. First, those who visit healthcare facilities are more likely to be enrolled in the study, and these participants will likely experience better survival. Additionally, the younger onset cases often spark more interest and are more likely to be referred and/or participate in the study than older patients, in whom PD can often go unnoticed or deemed insignificant relative to other maladies. Furthermore, participants from rural areas were underrepresented. This low penetration in rural communities is likely due to the lack of movement disorder specialization; participants must travel to metropolitan areas to first learn about the study. Thus, the cohort presented in this study is not indicative of all people with PD and may suffer from selection bias with limited representation of rural communities.
- However, the present study has important strengths. First, it is one of the largest studies of its kind with 1,334 participants and 1,127 controls. Many other studies investigating mortality in PD have fewer than 500 participants, with numbers usually ranging from 100 to 300 individuals. Thus, potential relationships are less likely to be impacted in a small participant population, a drawback of many other studies [3,4,8,20,33]. Furthermore, because this study is an ongoing project, 20 years of data are available for evaluation, allowing for a more comprehensive analysis of PD mortality and other relevant aspects of progression.
- In conclusion, our analyses of the considerable amount of QPP data reveal significantly increased mortality in PD. The age at death was only moderately reduced compared with the general population of Queensland, Australia. Age at onset and sex seem to be consistent independent predictors of mortality, and DBS treatment is associated with reduced mortality. Survival in PD is longer than that in most previous reports. Expanding the data with more consistent follow-up information should provide more in-depth insights into the factors associated with progression and mortality in PD.
DISCUSSION
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Author Contributions
Conceptualization: Peter Poortvliet, George Mellick. Data curation: Peter Poortvliet. Formal analysis: Alexander Gluch. Investigation: Peter Poortvliet, Peter Silburn, George Mellick. Methodology: Alexander Gluch, Peter Poortvliet. Project administration: George Mellick, Peter Poortvliet. Writing—original draft: Alexander Gluch, Peter Poortvliet. Writing—review and editing: all authors.
-
Ethical Standards
This study is an overview of 20 years of data collection of the Queensland Parkinson’s Project, an ongoing, ethically approved project aimed at determining risk and progression factors in Parkinson’s disease in Australia. All procedures performed in the project were in accordance with the ethical standards of the institutional and/or national research ethics committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards.
Notes
- This study was supported by Griffith University.
Acknowledgments
| Sex | Queensland SMR (95% CI) | p | Australia SMR (95% CI) | p |
|---|---|---|---|---|
| Overall | ||||
| Female | 2.84 (2.47–3.22) | 0.001† | 2.87 (2.49–3.25) | 0.001† |
| Male | 2.41 (2.17–2.64) | 0.001† | 2.47 (2.23–2.72) | 0.001† |
| Total | 2.70 (2.49–2.91) | 0.001† | 2.75 (2.53–2.96) | 0.001† |
| Age of onset < 60 years | ||||
| Female | 3.91 (3.13–4.69) | 0.001† | 3.97 (3.18–4.77) | 0.001† |
| Male | 2.90 (2.45–3.43) | 0.001† | 2.98 (2.52–3.43) | 0.001† |
| Total | 3.47 (3.05–3.90) | 0.001† | 3.47 (3.05–3.90) | 0.001† |
| Age of onset ≥ 60 years | ||||
| Female | 2.36 (1.95–2.77) | 0.001† | 2.37 (1.96–2.79) | 0.001† |
| Male | 2.17 (1.89–2.44) | 0.001† | 2.23 (1.95–2.51) | 0.001† |
| Total | 2.36 (2.12–2.60) | 0.001† | 2.40 (2.15–2.64) | 0.001† |
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI)a | p | HR (95% CI)b | p | |
| Male sex | 1.40 (1.13–1.58) | 0.001† | 1.31 (1.09–1.56) | 0.003† |
| Age at onset | 1.11 (1.10–1.12) | 0.001† | 1.11 (1.10–1.12) | 0.001† |
| Neurosurgery | ||||
| DBS | 0.73 (0.56–0.93) | 0.012* | 0.76 (0.59–0.98) | 0.038* |
| Pallidotomy | 0.72 (0.39–1.32) | 0.283 | 0.74 (0.39–1.14) | 0.363 |
| Pesticide exposure | ||||
| Occasional | 1.52 (1.20–1.93) | 0.001† | 1.48 (1.17–1.88) | 0.001† |
| Regular | 1.21 (0.98–1.50) | 0.074 | 1.20 (0.97–1.49) | 0.098 |
| Smoking | 1.00 (0.85–1.18) | 0.984 | 1.02 (0.86–1.20) | 0.829 |
| Depression | 0.97 (0.82–1.14) | 0.679 | 0.99 (0.84–1.16) | 0.853 |
| Family history | 0.91 (0.76–1.09) | 0.295 | 0.90 (0.75–1.09) | 0.292 |
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI)a | p | HR (95% CI)b | p | |
| Male sex | 1.05 (0.81–1.35) | 0.738 | 0.85 (0.62–1.17) | 0.326 |
| Pesticide exposure | ||||
| Occasional | 2.58 (1.77–3.77) | 0.001† | 2.83 (1.90–4.22) | 0.001† |
| Regular | 1.14 (0.77–1.69) | 0.524 | 1.21 (0.81–1.82) | 0.351 |
| Smoking | 1.09 (0.82–1.46) | 0.554 | 1.19 (0.87–1.62) | 0.273 |
| Depression | 0.88 (0.58–1.31) | 0.546 | 0.84 (0.56–1.28) | 0.422 |
| Family history | 0.94 (0.70–1.26) | 0.675 | 0.94 (0.69–1.29) | 0.710 |
- 1. Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015;386:896–912.ArticlePubMed
- 2. Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 2014;29:1615–1622.ArticlePubMed
- 3. Diem-Zangerl A, Seppi K, Wenning GK, Trinka E, Ransmayr G, Oberaigner W, et al. Mortality in Parkinson’s disease: a 20-year follow-up study. Mov Disord 2009;24:819–825.ArticlePubMed
- 4. Hely MA, Morris JG, Traficante R, Reid WG, O’Sullivan DJ, Williamson PM. The Sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry 1999;67:300–307.ArticlePubMedPMC
- 5. Hobson P, Meara J, Ishihara-Paul L. The estimated life expectancy in a community cohort of Parkinson’s disease patients with and without dementia, compared with the UK population. J Neurol Neurosurg Psychiatry 2010;81:1093–1098.ArticlePubMed
- 6. Pinter B, Diem-Zangerl A, Wenning GK, Scherfler C, Oberaigner W, Seppi K, et al. Mortality in Parkinson’s disease: a 38-year follow-up study. Mov Disord 2015;30:266–269.ArticlePubMed
- 7. de Lau LM, Verbaan D, Marinus J, van Hilten JJ. Survival in Parkinson’s disease. Relation with motor and non-motor features. Parkinsonism Relat Disord 2014;20:613–616.ArticlePubMed
- 8. Zhang Y, Wang C, Wang Y, Xiao Q, Liu J, Ma J, et al. Mortality from Parkinson’s disease in China: findings from a ten-year follow up study in Shanghai. Parkinsonism Relat Disord 2018;55:75–80.ArticlePubMed
- 9. Australian Bureau of Statistics. Deaths, Australia, 2018 [Internet]; Canberra: Australian Bureau of Statistics; [cited 2020 July]. Available from: https://www.abs.gov.au/AUSSTATS/abs@.nsf/mf/3302.0.
- 10. Dissanayaka NN, Sellbach A, Matheson S, O’Sullivan JD, Silburn PA, Byrne GJ, et al. Anxiety disorders in Parkinson’s disease: prevalence and risk factors. Mov Disord 2010;25:838–845.ArticlePubMed
- 11. Wan Y, Zhu Y, Luo Y, Han X, Li Y, Gan J, et al. Determinants of diagnostic latency in Chinese people with Parkinson’s disease. BMC Neurol 2019;19:120.ArticlePubMedPMC
- 12. Breen DP, Evans JR, Farrell K, Brayne C, Barker RA. Determinants of delayed diagnosis in Parkinson’s disease. J Neurol 2013;260:1978–1981.ArticlePubMed
- 13. Australian Institute of Health and Welfare. Deaths in Australia [Internet]; Canberra: Australian Institute of Health and Welfare; 2020 [cited 2020 July]. Available from: https://www.aihw.gov.au/reports/life-expectancy-death/deaths-in-australia.
- 14. Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Rudolph A, et al. Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology 2005;64:87–93.ArticlePubMed
- 15. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844.ArticlePubMed
- 16. Morgan JC, Currie LJ, Harrison MB, Bennett JP Jr, Trugman JM, Wooten GF. Mortality in levodopa-treated Parkinson’s disease. Parkinsons Dis 2014;2014:426976.ArticlePubMedPMC
- 17. Schrag A, Ben-Shlomo Y, Brown R, Marsden CD, Quinn N. Young-onset Parkinson’s disease revisited--clinical features, natural history, and mortality. Mov Disord 1998;13:885–894.ArticlePubMed
- 18. Santos-García D, Mir P, Cubo E, Vela L, Rodríguez-Oroz MC, Martí MJ, et al. COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015), a global--clinical evaluations, serum biomarkers, genetic studies and neuroimaging--prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol 2016;16:26.PubMedPMC
- 19. Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. What predicts mortality in Parkinson disease? A prospective population-based long-term study. Neurology 2010;75:1270–1276.ArticlePubMed
- 20. Auyeung M, Tsoi TH, Mok V, Cheung CM, Lee CN, Li R, et al. Ten year survival and outcomes in a prospective cohort of new onset Chinese Parkinson’s disease patients. J Neurol Neurosurg Psychiatry 2012;83:607–611.ArticlePubMed
- 21. Hitti FL, Ramayya AG, McShane BJ, Yang AI, Vaughan KA, Baltuch GH. Long-term outcomes following deep brain stimulation for Parkinson’s disease. J Neurosurg 2019;132:205–210.Article
- 22. Ngoga D, Mitchell R, Kausar J, Hodson J, Harries A, Pall H. Deep brain stimulation improves survival in severe Parkinson’s disease. J Neurol Neurosurg Psychiatry 2014;85:17–22.ArticlePubMed
- 23. Schüpbach MW, Welter ML, Bonnet AM, Elbaz A, Grossardt BR, Mesnage V, et al. Mortality in patients with Parkinson’s disease treated by stimulation of the subthalamic nucleus. Mov Disord 2007;22:257–261.ArticlePubMed
- 24. Lilleeng B, Brønnick K, Toft M, Dietrichs E, Larsen JP. Progression and survival in Parkinson’s disease with subthalamic nucleus stimulation. Acta Neurol Scand 2014;130:292–298.ArticlePubMed
- 25. Toft M, Lilleeng B, Ramm-Pettersen J, Skogseid IM, Gundersen V, Gerdts R, et al. Long-term efficacy and mortality in Parkinson’s disease patients treated with subthalamic stimulation. Mov Disord 2011;26:1931–1934.ArticlePubMed
- 26. Poortvliet PC, Silburn PA, Coyne TJ, Chenery HJ. Deep brain stimulation for Parkinson disease in Australia: current scientific and clinical status. Intern Med J 2015;45:134–139.ArticlePubMed
- 27. Hartmann CJ, Fliegen S, Groiss SJ, Wojtecki L, Schnitzler A. An update on best practice of deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord 2019;12:1756286419838096.ArticlePubMedPMC
- 28. Ritz B, Yu F. Parkinson’s disease mortality and pesticide exposure in California 1984-1994. Int J Epidemiol 2000;29:323–329.ArticlePubMed
- 29. Brouwer M, Koeman T, van den Brandt PA, Kromhout H, Schouten LJ, Peters S, et al. Occupational exposures and Parkinson’s disease mortality in a prospective Dutch cohort. Occup Environ Med 2015;72:448–455.ArticlePubMed
- 30. Tomenson JA, Campbell C. Mortality from Parkinson’s disease and other causes among a workforce manufacturing paraquat: a retrospective cohort study. BMJ Open 2011;1:e000283. ArticlePubMedPMC
- 31. Moscovich M, Boschetti G, Moro A, Teive HAG, Hassan A, Munhoz RP. Death certificate data and causes of death in patients with parkinsonism. Parkinsonism Relat Disord 2017;41:99–103.ArticlePubMed
- 32. Keener AM, Paul KC, Folle A, Bronstein JM, Ritz B. Cognitive impairment and mortality in a population-based Parkinson’s disease cohort. J Parkinsons Dis 2018;8:353–362.ArticlePubMedPMC
- 33. Montastruc JL, Desboeuf K, Lapeyre-Mestre M, Senard JM, Rascol O, Brefel-Courbon C. Long-term mortality results of the randomized controlled study comparing bromocriptine to which levodopa was later added with levodopa alone in previously untreated patients with Parkinson’s disease. Mov Disord 2001;16:511–514.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Update: Descriptive epidemiology of Parkinson disease
Nikolas Grotewold, Roger L. Albin
Parkinsonism & Related Disorders.2024; 120: 106000. CrossRef - Indication for molecular testing by multiplex ligation‐dependent probe amplification in parkinsonism
E. Mutez, M. Swiderski, D. Devos, C. Moreau, G. Baille, A. Degardin, G. Ryckewaert, N. Carriere, A. Kreisler, C. Simonin, N. Rouaix, M. Tir, P. Krystkowiak, N. Ramdane, M. Génin, B. Sablonnière, L. Defebvre, V. Huin
European Journal of Neurology.2023; 30(6): 1667. CrossRef - Different pieces of the same puzzle: a multifaceted perspective on the complex biological basis of Parkinson’s disease
Amica C. Müller-Nedebock, Marieke C. J. Dekker, Matthew J. Farrer, Nobutaka Hattori, Shen-Yang Lim, George D. Mellick, Irena Rektorová, Mohamed Salama, Artur F. S. Schuh, A. Jon Stoessl, Carolyn M. Sue, Ai Huey Tan, Rene L. Vidal, Christine Klein, Soraya
npj Parkinson's Disease.2023;[Epub] CrossRef - Effects of omega-3 polyunsaturated fatty acids on the levels of pro- and anti-inflammatory cytokines and lipid profile in patients with Parkinson's disease
Sara Mohammadi, Mirmohsen Sharifi Bonab, Mahdyieh Hamed Behzad, Bahram Pourghassem Gargari
Nutrition Clinique et Métabolisme.2023; 37(3): 181. CrossRef - The elephant in the room: critical reflections on mortality rates among individuals with Parkinson’s disease
Lisanne J. Dommershuijsen, Sirwan K. L. Darweesh, Yoav Ben-Shlomo, Benzi M. Kluger, Bastiaan R. Bloem
npj Parkinson's Disease.2023;[Epub] CrossRef - Mortality of Parkinson’s disease in Italy from 1980 to 2015
Monica Ulivelli, Daiana Bezzini, Lucia Kundisova, Ilaria Grazi, Mario Alberto Battaglia, Nicola Nante, Simone Rossi
Neurological Sciences.2022; 43(6): 3603. CrossRef - A nationwide study of the incidence, prevalence and mortality of Parkinson’s disease in the Norwegian population
Brage Brakedal, Lilah Toker, Kristoffer Haugarvoll, Charalampos Tzoulis
npj Parkinson's Disease.2022;[Epub] CrossRef - Australian Parkinson’s Genetics Study (APGS): pilot (n=1532)
Svetlana Bivol, George D Mellick, Jacob Gratten, Richard Parker, Aoibhe Mulcahy, Philip E Mosley, Peter C Poortvliet, Adrian I Campos, Brittany L Mitchell, Luis M Garcia-Marin, Simone Cross, Mary Ferguson, Penelope A Lind, Danuta Z Loesch, Peter M Vissche
BMJ Open.2022; 12(2): e052032. CrossRef - Therapeutic targeting of mitophagy in Parkinson's disease
Shashank Masaldan, Sylvie Callegari, Grant Dewson
Biochemical Society Transactions.2022; 50(2): 783. CrossRef - Worldwide trends in mortality related to Parkinson's disease in the period of 1994–2019: Analysis of vital registration data from the WHO Mortality Database
Ioannis C. Lampropoulos, Foteini Malli, Olga Sinani, Konstantinos I. Gourgoulianis, Georgia Xiromerisiou
Frontiers in Neurology.2022;[Epub] CrossRef - Effects of physician visit frequency for Parkinson’s disease treatment on mortality, hospitalization, and costs: a retrospective cohort study
Takako Fujita, Akira Babazono, Sung-a Kim, Aziz Jamal, Yunfei Li
BMC Geriatrics.2021;[Epub] CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite