Gait Analysis in Patients With Parkinson’s Disease: Relationship to Clinical Features and Freezing
Article information
Abstract
Background:
The purpose of our study was to investigate gait dynamics and kinematics in patients with Parkinson’s disease (PD) and to correlate these features with the predominant clinical features and with the presence of the freezing of gait (FOG). We measured the temporospatial and kinematic parameters of gait in 30 patients with PD (M:F=12:18, age=68.43±7.54) using a computerized video motion analysis system.
Methods:
We divided the subjects into subgroups: (1) tremor-dominant (TD) group and postural instability and gait disturbance (PIGD) group and (2) FOG group and non-FOG group. We compared the gait parameters between the subgroups.
Results:
The walking velocity and stride length were reduced significantly in the PIGD group compared to the TD group. The PIGD group showed a significantly reduced range of motion in the pelvic and lower extremity joints by kinematics. Stride time variability was significantly increased and the pelvic oblique range was significantly reduced in the freezing gait disorder group.
Conclusion:
Our findings suggest that there are differences in the perturbation of the basal ganglia-cortical circuits based on major clinical features. The reduction of the pelvic oblique range of motion may be a compensatory mechanism for postural instability and contributes to stride time variability in patients with FOG.
INTRODUCTION
Patients with Parkinson’s disease (PD) often present with difficulty in initiating and/or maintaining normal walking. The disturbance of routine gait activity may cause substantial discomfort and impairment in activities of daily living. Gait requires a complex set of behaviors which include the simultaneous performance of locomotion while maintaining balance. Gait disturbance is a common feature of movement disorders, especially in PD. Careful observation and analysis of gait patterns frequently helps diagnose patients with movement disorders.[1]
PD is primarily a disturbance of motor function typically with bradykinesia, resting tremor and rigidity. Gait disturbance and postural instability have been recognized as important motor signs of PD. However, such clinical features do not appear in all patients with PD. Patients with PD can be classified by major clinical features. Jankovic et al. classified patients with PD as a tremor-dominant (TD) group or a postural instability and gait disturbance (PIGD) group.[2] About one-third of patients with PD have sudden and transient disturbance in motor performance, known as the ‘freezing phenomenon’.[3,4] Freezing of gait (FOG), occurs when a patient cannot walk forward, as if the feet were stuck to the ground. It is a unique symptom that frequently causes patients to fall. FOG is a common and disabling gait problem in patients with PD, especially in the advanced stages.[5] FOG, in off-phase PD, responds well to levodopa suggesting a dopa deficiency with resulting motor circuit dysfunction as the underlying pathophysiological mechanism; however, levodopa is not effective for treatment of FOG in advanced stage PD or in other parkinsonian syndromes. Recently, quantitative gait analysis studies suggested that FOG is caused by a combination of increased inability to generate stride length superimposed on an unregulated walking cadence.[6]
The main purpose of this study was to investigate the temporospatial and kinematic patterns of gait with a three dimensional gait analysis system in patients according to their clinical features comparing (1) the PIGD group with the TD group, and (2) the freezing group with the non-freezing group.
MATERIALS AND METHODS
1. Subjects
We recruited patients from the Movement Disorders Unit at the Department of Neurology at Korea University Guro Hospital. PD was defined according to the Clinical Diagnostic Criteria described by the United Kingdom Parkinson’s Disease Society Brain Bank.[7] We excluded patients who reported clinically significant co-morbid disorders likely to affect gait including: stroke, orthopedic disease, rheumatologic disease, cardiovascular disease and pulmonary disorders. Patients who could not perform the three dimensional gait analysis because of cognitive impairment (Mini Mental Status Examination < 24) were also excluded. The recruited patients participated in the gait analysis at least 24 hours after taking anti-Parkinson medication, while in the “off” status. Written informed consent was obtained from each patient for study participation. This study was approved by the Institutional Review Board at Korea University Guro Hospital.
An average global tremor score was calculated as the mean of the following items: right and left arm tremor, as determined by history; tremor of face, lips or chin at rest; tremor of all four limbs at rest; action or postural tremor in both arms as determined by the investigator’s examination. A mean score for the complex of postural instability and gait difficulty (PIGD) was calculated as the mean of the following items: falling, freezing, walking difficulty by history as well as gait and postural instability by examination. The tremor - dominant group (TD) included patients with a ratio of the mean tremor score/mean PIGD score greater than or equal to 1.5; the PIGD group included all patients with a ratio of less than or equal to 1.0.[2] Patients were divided into a freezing group and a non-freezing group based on clinical history and the UPDRS motor score.
2. Gait Protocols and Instrumentation
All gait evaluations were performed and recorded by an identical method. The standing posture was used as the baseline standard. Subjects were instructed to walk at their usual pace on level ground for 10 m, turn and walk the same route back. This was repeated until identical and complete walking information was obtained.
We performed the gait analysis with an automatic computerized video motion analysis system (Oxford Metrix vicon 512 motion analysis system). Based on biomechanical models, the pelvis and the lower extremities of each subject were measured by 15 retro-reflective markers. These markers were attached to the anterior superior iliac spine, lateral aspect of the thighs and shin, knee jointaxis, lateral malleoli, heels, forefeet and the sacrum area. A computerized system recorded the information from the retro-reflective markers automatically at a speed of 30 Hz. This information was then transferred to a workstation. The temporospatial parameters were reconstructed and analyzed automatically at the workstation. Stride time variability, the magnitude of the stride-to-stride fluctuations in the gait cycle duration, was calculated by determining the standard deviation and the coefficient of variation of each subject’s stride time.[8]
3. Data analysis
Spatiotemporal and kinematic parameters were collected and analyzed for the entire stride period. The maximal value of the amplitude for the motion of the pelvis, hip, knee and ankle were measured directly, and the range of motion of each joint was calculated. An independent t-test was used to compare each group (TD/PIGD and freezing group /non-freezing group). Statistical results with a p-value < 0.05 were considered significant. Statistical analysis was performed with Statistical Package for Social Science Software (version 10.0, SPSS, Chicago, IL) for Windows.
RESULTS
1. Demographic Factors
A total of 30 patients completed the study. Twelve males and 18 females had a mean age of 68.8±7.49 years. Hoehn and Yahr stage I included 10 patients, II had 11 patients, III had nine patients and IV had one patient. Among 30 patients, the TD group included nine patients (66.78±6.02, male : female = 4 : 5), and the PIGD group had 21 patients (69.14±8.14, male : female = 8 : 13). The freezing group included six patients (68.67±10.41, male : female = 3 : 3), and the non- freezing group had 24 patients (68.38±6.94, male : female = 9 : 15). The demographic and clinical characteristics of patients are summarized in Table 1.
2. The tremor-dominant patient (TD) group and the postural instability and gait disturbance-dominant patient (PIGD) group
1) Spatiotemporal results
The patients in the PIGD group had a significant reduction in walking velocity, stride length and ratio of single support time to double support time compared to the TD group. However, the cadence, single support time and double support time were not significantly different (Table 2).
2) Kinematic results
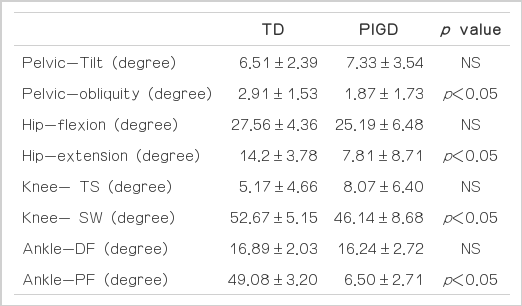
For the PIGD group, the range of pelvic oblique, hip extension, ankle plantar flexion and knee flexion during the swing phase (SW) were significantly reduced. However, the range of pelvic tilting, hip flexion and knee flexion during terminal stance (TS) and ankle dorsiflexion were not significantly different (Table 3).
3. The patient group with the freezing phenomenon and the patient group without the freezing phenomenon
1) Spatiotemporal results
The stride time variability was significantly increased in the freezing group. However, there were no significant differences in the walking velocity, walking speed, stride length, the single support time and the double support time between the freezing group and the non-freezing group (Table 4).
2) Kinematic results
In the freezing group, the pelvic oblique motion was significantly decreased. However, other kinematic parameters (pelvic tilting, range of motion of hip and ankle) were not significantly different (Table 5).
DISCUSSION
Patients with PD have distinctive features of decreased walking velocity, reduced stride length and intact control of cadence in their gait dynamics.[9,10] In addition, during a gait cycle, the ratio of double support time to single support time increases in patients with PD compared to normal controls. Previous studies have reported that stimulation of the internal globus pallidus mediates a limited affect on the control of stride length however, stimulation of the subthalamic nucleus has been shown to mediate a significant affect on stride length. Thus the role of the subthalamic nucleus in the control of stride length may be substantial.[11–13] Therefore, excessive activity in the subthalamic nucleus, which is observed in PD, may cause the abnormality in control of stride length. Morris et al. have reported that the basal ganglia do not control stride length directly, and that the motor cortical regions such as the primary motor cortex, the premotor cortex, and the supplementary motor areas determine the range and order of motion depending on the environment and work demands.[14,15] Functional imaging study has suggested a difference between tremor-dominant PD and PIGD-dominant PD with regard to regional patterns of blood flow reduction in the brain.[16] In our study, the walking cadence of the PIGD group compared to the TD group was not altered; nonetheless, a noticeable reduction of the walking velocity and stride length could be detected. With these results, we would predict a difference in the pattern of perturbation in the basal ganglia-cortical circuits, especially in the subthalamic nucleus, between tremor-dominant PD and PIGD-dominant PD.
In mild cases of PD, freezing of gait (FOG) is found in approximately seven percent of patients. Fifty percent of FOG patients have more advanced PD.[17] The mechanisms underlying FOG has not been characterized to date. Nieuwboer et al. has suggested that gait disturbance could be detected prior to the development of the freezing phenomenon.[18] Hausdorff et al. suggested that PD patients with FOG had a continuous gait disturbance: an inability to regulate the stride-to-stride variations in gait timing. In our study, the stride time variability, which may suggest gait irregularity and instability, was increased in the freezing group.[19] These results suggest that FOG does not suddenly develop but rather is a progressive gait disorder that reflects damage to the control of the stride to stride gait fluctuation. Therefore, we could detect gait disturbances between episodes of the freezing phenomenon, and during the freezing phenomenon with a computerized three dimensional gait analysis system. In addition, we observed that the pelvic oblique movements were reduced in the freezing group. This was thought to be a compensatory mechanism to overcome the freezing phenomenon and to maintain postural stability.
In conclusion, we detected changes in walking velocity and stride length and decreased range of motion in the PIGD group. This was thought to be caused by abnormal regulation of the cerebral cortex-basal ganglia. Stride timee variability was increased and the pelvic oblique range of motion was decreased in the freezing group. It is thought that FOG is not a distinct phenomenon but rather a type of gait disorder, with the loss of the ability to control changes of stride length, to compensate for such changes and to maintain postural stability.