Paroxysmal Chorea as a Relapse of Myelopathy in a Patient with Neuromyelitis Optica
Article information
Abstract
Movement disorders secondary to intrinsic spinal cord disease are rare. Paroxysmal chorea has not yet been reported in the neuromyelitis optica (NMO). We report a 43-year-old woman with relapsing-remitting cervical myelopathy who developed paroxysmal chorea during clinical exacerbation of NMO. MRI scan of the cervical spine revealed a long segmental enhancing lesion, but brain MRI did not show any responsible abnormalities. Acute exacerbation of recurrent myelopathy in NMO may be associated with transient movement disorder.
Movement disorders secondary to intrinsic spinal cord disease are rare but can be caused by neoplastic, inflammatory, demyelinative, or traumatic lesions.1–3 Paroxysmal chorea, one of a variety of involuntary movements associated with spinal cord disease, is extremely rare. Here we describe a case of paroxysmal chorea associated with recurrent cervical myelopathy. Long-term clinical follow-up confirmed that the patient had a neuromyelitis optica (NMO).
Case Report
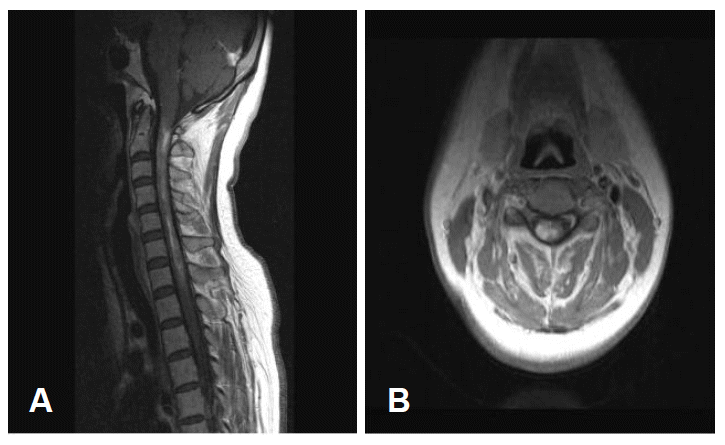
A 43-year-old woman presented with recurrent paroxysmal involuntary movements of her right upper limb and quadriparesis. She had four previous relapsing and progressive episodes presenting as right upper limb monoparesis, quadriparesis, and paresthesias during three years. The previous weakness resolved incompletely with steroid treatment. On admission, her wide right forearm-flinging movements, which were occasionally provoked by left knee flexion, were associated with distal choreic movements. Lying on the bed, the attacks involved flexion of the right elbow, repeated flexion and extension of the ipsilateral wrist. It appeared as though she was playing the drums. The duration of each episode was approximately 15–30 seconds. Her only medication was baclofen, which she had been taking for the last three years. She had a quadriparesis (grade 3–4, Medical Research Council), worse on the right limbs and distal muscles. Rough touch, pain, and temperature sensation were slightly decreased on all extremities, but there were no deep sensory abnormalities. Deep tendon reflexes were very brisk and the plantar response was extensor bilaterally. Laboratory studies including routine chemistries, various serological markers related with connective tissue diseases, thyroid function tests, and serum and urine copper levels were all normal. CSF examination showed mild elevation of protein (80.5 mg/dL) and IgG index (0.70), but oligoclonal bands and NMO-IgG antibodies were negative. Electroencephalography and visual evoked potentials revealed no abnormalities. Median and posterior tibial nerve somatosensory evoked potentials showed no N13, N20 and P37. MRI revealed diffuse swelling and heterogeneous enhancement of spinal cord from the cervicomedullary junction to C7 (Figure 1). Repeated brain MRIs, taken whenever quadriparesis occurred, showed no responsible lesion. She was treated with steroid and clonazepam. Choreic movements gradually disappeared over 1 week. The weakness improved slowly over a month and she could walk with a cane. Six months after the disappearance of involuntary movement, she developed decreased visual acuity with prolonged latencies of P100 components in visual evoked potentials. Her vision gradually improved over the next few weeks.
Discussion
The occurrence of ballism-chorea and dystonia implies development of lesions involving the basal ganglia and their afferent or efferent connections, such as thalamus, subthalamic nucleus, basal ganglia, and putamen. However, the unique feature of this patient is choreic movement without responsible brain lesions. The flinging movement of her right arm and forearm even resembled ballism. Ballism has never been reported in spinal cord lesions whereas chorea has been seen very rarely.4 It was uncertain whether she exhibited a pure ballism or chorea but presumably it is not important. While ballism and chorea are distinguishable based upon the type and distribution of movements, they may represent two different symptoms or a spectrum of the same basic disease processes.5 Why a patient with a spinal cord lesion develops chorea but not ballism is unknown. Ballism, a coarse proximal movement, could evolve into more distal and lower amplitude chorea. Athetosis, which is considered a slow form of chorea, often evolves further into dystonia.5 Why movement disorders occur in only a minority of patients with myelopathy is also unknown. A number of different mechanisms have been suggested, but were poorly understood. One mechanism involves the posterolateral cervical cord, the projections from supraspinal centers on the Ia inhibitory neurons, and other interneurons concerned with reciprocal inhibition.1–3 Disruption of the somatosensory pathways or motor cortex to the striatum also may produce abnormal movement without sensory loss.1 For our purposes, considering paroxysmal chorea and dystonia together as involuntary movements, we can infer the effect of provocative movements in this patient from the results of previous studies. Paroxysmal chorea could be easily provoked by movement, either passive or volitional, and by tactile stimulation.4 It often crosses to the contralateral trunk or limbs. The so called trigger zone was found at either the same spinal segment or below the clinically estimated segment.
Although this patient had relapsing-remitting weakness, it did not fulfill the conditions of definite NMO when the involuntary movements occurred. However, she must have had NMO, considering the visual symptoms afterwards. Despite the lack of lesions located appropriately for the involuntary movements, steroid therapy was suggested in relapsing-remitting NMO. However, we could not evaluate the effectiveness of such therapy because our patient was treated with steroid and clonazepam.
Movement disorders associated with multiple sclerosis or NMO are rare. Our case suggests that ballism-chorea could present in a spinal cord lesion without involvement of striatum and deep sensory abnormalities. Thus, acute exacerbation of recurrent myelopathy in NMO may be associated with transient movement disorders.