Hemidystonia as an Initial Manifestation of Leptomeningeal Metastasis
Article information
Abstract
A 76-year-old woman gradually developed action dystonia of the left hand and foot. Leptomeningeal metastasis of the right fronto-parietal area associated with gastric adenocarcinoma was found on the brain magnetic resonance imaging (MRI) and positron emission tomography (PET) studies. We discuss the mechanisms involved in the development of secondary hemidystonia and review dystonia associated with cortical lesions.
Focal or hemidystonia usually appears as a consequence of lesions in the contralateral basal ganglia and thalamus.1 Reports of cases with hemidystonia emphasize the role of the basal ganglia in the genesis of secondary dystonia. Recently several cases of secondary dystonia due to cortical lesions have been reported.2 Cortical lesions resulting in dystonia were usually described as destructive,2 and in such cases the dystonia frequently develops as a delayed phenomena.3,4 Leptomeningeal metastasis (LM) is a complication of cancer. The neurological signs and symptoms of LM are associated with the involved neuraxis.5 A case of action myoclonus was reported as an early clinical sign of leptomeningeal carcinomatosis.6 However, other movement disorders such as dystonia have not yet been reported.
We report a patient presenting with hemidystonia as the initial manifestation of leptomeningeal metastasis associated with gastric adenocarcinoma.
Case Report
A 76-year-old woman was admitted to our hospital because of involuntary movement of the left distal extremities. Two months earlier, she reported a peculiar feeling and occasional jerky and twisting movements of the left foot and hand when attempting to put on shoes or catch objects. The symptoms worsened over the next two months making fine motor tasks difficult. The patient had a past history of hypertension and endometrial cancer that was treated.
On initial examination, we found dystonic movement of the left hand and foot mainly occurring with action. On neurological examination, motor and sensation functions were preserved, but there were increased deep tendon reflexes, a positive Barbinski’s reflex and ankle clonus in the left extremities. The gait was small based and dystonia of the left foot was observed on initiation of gait.
The brain MRI revealed hyperintense lesions with irregular and nodular meningeal enhancement in the right fronto-parietal lobe, suggesting leptomeningeal metastasis (Figure 1). The MR angiography, venography and spectroscopy were non-specific. The electroencephalogram for a differential diagnosis of simple partial seizure showed no epileptic activity. The cerebrospinal fluid (CSF) contained 54 white blood cells per uL (47% lymphocyte dominant); the concentrations of protein and glucose were 79 mg/dL and 65 mg/dL, respectively. All laboratory tests including tumor marker studies and CSF cytology were normal.

Brain MR showed hyperintensity and focal thickening of the right superior, middle frontal gyri and postcentral gyrus on the T2-weighted image (black arrow)(A) and fluid attenuated inversion recovery (FLAIR) image (B). Enhanced brain MR showed irregular and nodular enhancement of the right precentral and central sulci and meningeal enhancement along the right interhemispheric fissure (black arrow head)(C and F). T2-weighted and FLAIR image of the basal ganglia and thalamus showed no significant abnormal findings except for multiple small vessel disease (D and E).
The search for a hidden primary malignancy included a whole body 18F-fluorodeoxyglucose positton emission tomography (18F-FDG PET). Torso-PET scanning showed hypermetabolic signals in the gastric antrum and left upper lung, and the brain-PET scan showed hypermetabolic signals in the right fronto-parietal lobe, consistent with the brain MRI findings (Figure 2). Endoscopic gastroduodenoscopy revealed a very large ulcerated infiltrating mass at the gastric antrum, and the biopsy showed an adenocarcinoma. Finally, the patient was diagnosed with advanced gastric cancer and leptomeningeal metastasis.
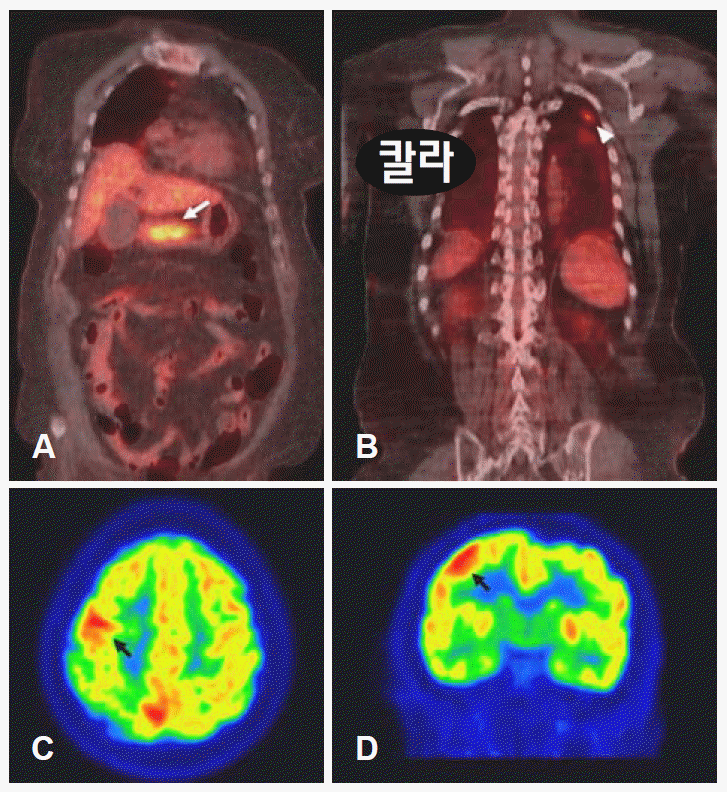
Torso FDG-PET showed hypermetabolic activity (maximum SUV: 5.7) of the gastric antrum (white arrow)(A) and left upper lobe of the lung (maximum SUV: 5.5)(white arrow head)(B). Brain FDG-PET showed hypermetabolic activity (maximum SUV: 10.2) of the right superior and middle frontal gyri and part of the interhemispheric fissure (black arrow)(C and D). FDG-PET: fluorodeoxygclucose positton emission tomography.
The patient received chemotherapy and medication with haloperidol, but the dystonia persisted. Two months later, after changing medication with clonazepam, the dystonia was slightly improved. Follow up brain MR continued to show hyperintense lesions without interval change.
Discussion
Basal ganglia dysfunction, with an imbalance between normal modulation of the direct and indirect pathways, is generally considered the basis for cortical disinhibition and abnormal motor output in the pathophysiology of dystonia.7 Consequently, secondary dystonia is usually produced by lesions in the contralateral caudate nucleus, lentiform nucleus or thalamus, or in a combination of these structures.1 These lesions are thought to cause the abnormal functioning of the cortico-striato-pallido-thalamo-cortical loop leading to enhanced excitation of the premotor cortical area.8 However, recent studies have emphasized the role of abnormal cortical function, especially in the somatosensory system, for the development of dystonia.9 Chuang and colleagues10 described that cortical lesions could be seen in up to 32% of patients with acquired hemidystonia. We identified 14 cases of symptomatic dystonia due to cortical lesions in the literature (Table 1).2,11–18
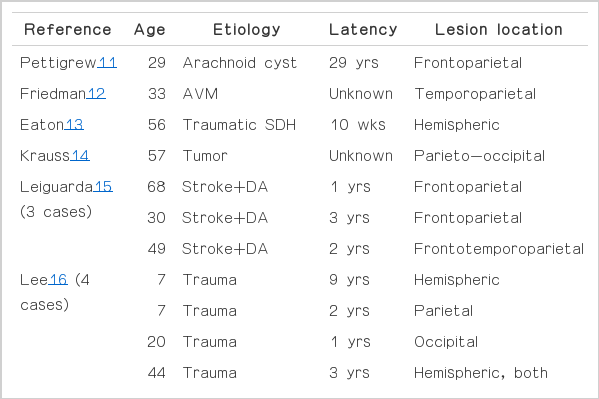
Review of the literatures about secondary dystonia due to cortical lesion: 14 cases from 9 references
There is often a latency between cerebral injury and the onset of dystonia, and such delayed development of dystonia might be related to aberrant reorganization of the cortico-striato-pallido-thalamo-cortical loop after a static lesion.3,10 Though most cases of secondary dystonia due to stroke or trauma develop after a long latency from the initial brain lesion, a few cases of secondary dystonia due to neoplasm have presented as the initial manifestation of cortical lesion, similar to our case.14 Functional neuroimaging showed overactivation of the primary sensorimotor cortex and underactivation of the prefrontal motor areas with focal hand dystonia.19 It is likely that hypermetabolism in the primary sensorimotor cortex, in our case, could have played a role in the genesis of dystonia. One prior report suggested that provoking the cerebral cortex can evoke dystonia in the extremities.19
Clinical signs and symptoms of LM are usually attributable to the obstruction of normal CSF flow, local tumor infiltration into the brain or spinal cord, alterations in the metabolism of underlying nervous tissues, or a combination of these processes. The common presenting signs of LM are headache, changes in mental status, focal neurological deficits, and seizures.5 There have been few reports on movement disorders such as dystonia secondary to LM. Our patient presented with left hand and foot dystonia, as the initial clinical manifestation of leptomeningeal metastasis associated with gastric adenocarcinoma. This is the first case described in which hemidystonia was attributed to leptomeningeal metastasis.