Sex and Gender Influence Urinary Symptoms and Management in Multiple System Atrophy
Article information
Abstract
Objective
Multiple system atrophy (MSA) is characterized by urinary dysfunction, yet the influence of sex and gender on urinary symptoms and treatment is unclear. We sought to characterize sex and gender differences in the symptomatology, evaluation, and management of urinary dysfunction in patients with MSA.
Methods
Patients with MSA evaluated at our institution were reviewed and stratified by sex.
Results
While the prevalence of urinary symptoms was similar in male and female patients, incontinence was more common in females. Despite this, males and females underwent postvoid residual (PVR) measurement at similar rates. While catheterization rates were similar when PVR was measured, males were more than twice as likely to be catheterized than females in the absence of PVR measurement.
Conclusion
Urinary symptoms are common in MSA, but their presentation differs between males and females. The difference in catheterization rates may be driven by a gender disparity in referrals for PVR, which can guide treatment.
Multiple system atrophy (MSA) is a neurodegenerative disease characterized by parkinsonism, cerebellar ataxia, and autonomic failure. [1]. Urinary failure is often a prominent and early symptom of MSA [2] and is a negative predictor of survival. As such, it is prudent to recognize, evaluate, and treat urinary dysfunction in patients with MSA.
Over the last several decades, there has been increasing interest in the role of sex (a spectrum of biological and physiological differences traditionally characterized as male and female) and gender (socially constructed roles on a broad gender spectrum) in neurologic disease [3,4] While understanding sex differences in neurologic disorders is pertinent to identify risk factors and targets for diagnostic and therapeutic measures, characterizing the role of gender often elucidates differences in the approach to and delivery of health care [5-7], and the interplay of sex and gender is often seen in patient outcomes [8]. We sought to further characterize sex and gender differences influencing urinary dysfunction, assessment, and management in patients with MSA [9].
MATERIALS & METHODS
Study design
Patients with a diagnosis of probable or possible MSA [1] evaluated at our institution from January 1998–December 2012 were included. Only patients who permitted use of their clinical records for retrospective research were enrolled in our study. Neurologic and urologic provider documentation was reviewed to characterize urinary symptoms. All symptom onset was determined based on patient recollection of duration of symptoms as detailed in this documentation, and all patients completed standardized questionnaires regarding urinary incontinence, urgency and frequency. Postvoid residual (PVR) was obtained through ultrasound or catheter at the discretion of the urologic procedure team. Documentation from emergency department visits, admissions, general practitioner evaluation, consulting services, and ancillary staff was reviewed. This study was approved by the Institutional Review Board of Mayo Clinic (#12-008800) and informed consent was received for all patients.
Statistical analysis
Data were analyzed using R-based BlueSky Statistics software (BlueSky, Chicago, IL, USA). Categorical variables were analyzed using chi-square tests, while continuous variables were analyzed with unpaired t tests. PVR and time to catheterization data demonstrated a positively skewed distribution and were transformed on a logarithmic scale for analysis under a normal distribution. Analysis of the time to catheterization by sex was calculated using a Kaplan‒Meier time to event analysis. Statistical significance was set at p < 0.05.
RESULTS
Demographics
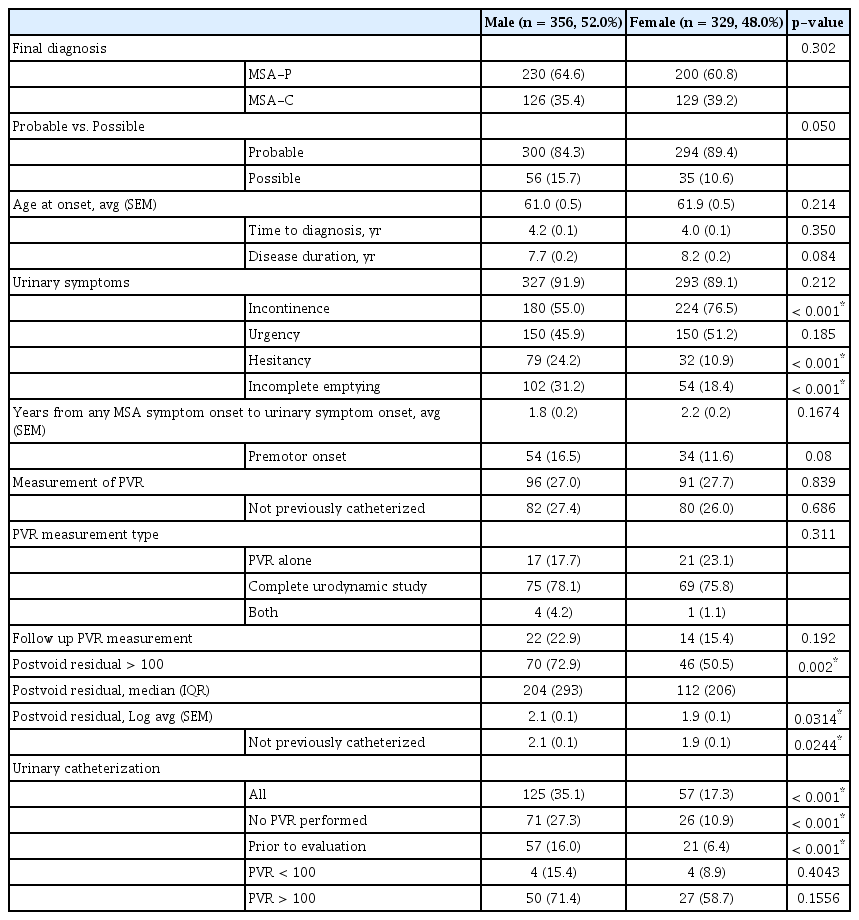
The cohort of patients evaluated at our institution with a diagnosis of MSA between 1998 to 2012 has been described previously and includes 356 males (52.0%) and 329 females (48.0%) (Table 1) [10]. Most patients (65.0%) had a follow-up period at their referring center of less than 90 days. There was no sex difference in age at symptom onset, years from symptom onset to diagnosis, disease duration, or MSA subtype (Table 1).
We found that 41% of males had a diagnosis of benign prostatic hypertrophy (BPH). Prior to diagnosis, 87 males underwent procedures to address urinary symptoms, and 82 (94.3%) of these procedures were directed at suspected prostatic involvement. By 3 months, the urinary symptoms of just 22 males had improved, and 10 of these 22 (11.5% of the entire cohort who underwent procedures) had no issues beyond 3 months. The urinary symptoms of 54 males who underwent procedures failed to improve or worsened immediately, with an overall failure in 71 (81.6% of the 87 male patients who underwent a procedure).
In contrast to males, only 49 female patients underwent procedures to address urinary symptoms (p = 0.001), many of which were directed at pelvic floor physiology. Of those who underwent procedures, symptoms failed to improve or recurred in 45 (91.8%).
Urinary symptoms
The proportion of males and females reporting urinary symptoms in their disease course to their neurologist during an office visit was similarly high (91.9% vs. 89.1%, respectively, p = 0.212) (Figure 1A). The average time from MSA diagnosis to bladder symptom onset did not differ between males and females (1.8 vs. 2.2 ± 0.2 years, p = 0.17), and there was no sex difference in the proportion of patients who developed bladder symptoms prior to the onset of motor findings (males, 16.5% vs. females 11.6%, p = 0.08). Other autonomic symptoms of this cohort have been described previously [10].
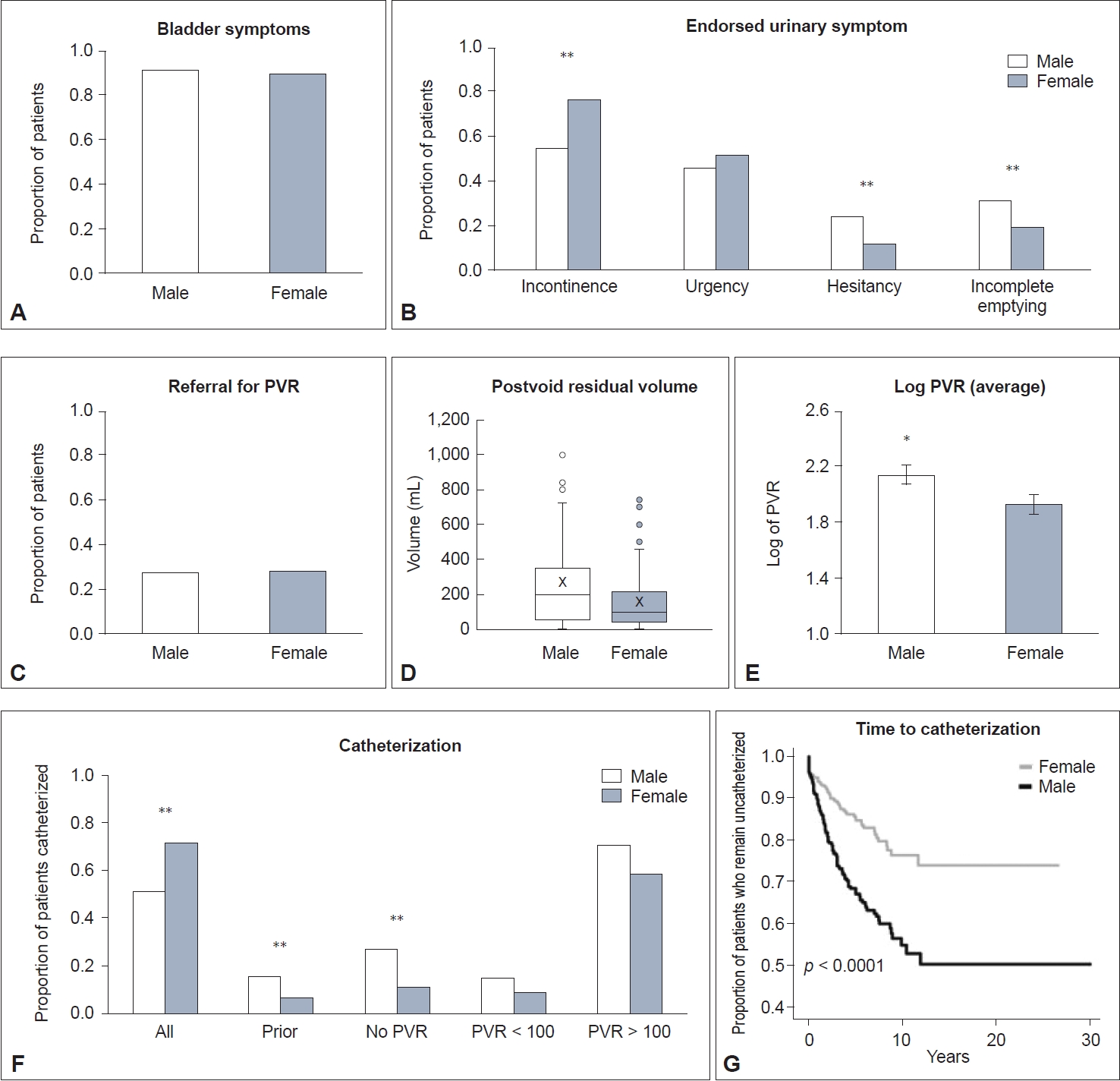
Sex and gender in urinary symptoms of multiple system atrophy (MSA). Proportion of patients with MSA (A) endorsing urinary symptoms, (B) endorsing incontinence, urgency, hesitancy, and incomplete emptying and (C) referred for measurement of postvoid residual (PVR) volume. D: Median (and interquartile range) PVR measured for males and females. The “x” represents the mean. E: Average (± SEM) logarithmic PVR for males and females. F: Proportion of patients with MSA catheterized overall, patients who were catheterized prior to presentation, those who did not undergo PVR measurement, and those who underwent measurement and had either normal (< 100) or elevated (> 100) PVRs. G: Kaplan‒Meier time to catheterization from onset of urinary symptoms. *p < 0.05; **p < 0.001. SEM, standard error of the mean.
More females endorsed incontinence (68.1%) than males (50.6%; p < 0.001), while males endorsed hesitancy (24.2% vs. 10.9%, p < 0.001) and incomplete emptying (31.2% vs. 18.4%, p < 0.001) more frequently than females. There was no sex difference in urgency (Figure 1B).
Measurement of postvoid residual
The proportion of males versus females who underwent PVR measurement was equal (27.0% vs. 27.7%, p = 0.839) (Figure 1C). The distribution of time to measurement of PVR from onset of urinary symptoms for both males and females was skewed to the right; over 40% of all evaluated patients underwent their evaluation within the first 18 months of symptoms (median time 1.5 years for males vs. 1.7 in females). Males and females who were already catheterized accounted for 14.6% and 12.1% of PVR measurements for their groups, respectively, and removal of their measurements from the analysis did not significantly change statistical findings. Thirty-eight patients had PVR measured by ultrasound, while 144 patients underwent catheterized PVR measurement. There was no sex difference in whether PVR was obtained with ultrasound or by catheterized samples (p = 0.311).
Males were significantly more likely to have an elevated PVR (> 100 mL) than females (72.9% vs. 50.5%, p = 0.002) (Figure 1D). Males also had a larger average log-transformed PVR (males, 2.1 ± 0.1 vs. females, 1.9 ± 0.1; p = 0.0314) (Figure 1E). There was no difference in average log-transformed PVR among males without or with a comorbid diagnosis of BPH (2.1± 0.1 vs. 2.2 ± 0.1; p = 0.39; data not shown).
Catheterization
Catheterization rates were significantly lower in patients who were followed up for less than 90 days than in those who were followed up for 90 days or more (22.5% vs. 34.2, p < 0.001). However, there was no difference in the average log-transformed time to catheterization from the onset of urinary symptoms (< 90 days, 0.19 ± 0.05 vs. ≥ 90 days, 0.26 ± 0.06 years, p = 0.3204).
While 16.0% of male patients were catheterized before evaluation at our institution, only 6.4% of females were already catheterized (p < 0.001). Overall, males were significantly more likely to be catheterized than females (35.1% vs. 17.3%, p < 0.001) (Figure 1F). When stratified by PVR, catheterization rates were similar between males and females when PVR was normal (15.4% vs. 8.9%, p = 0.4043) and when PVR was elevated (71.4% vs. 58.7%, p = 0.1556). When no PVR was measured (males, n = 260 vs. females, n = 238), males were more than twice as likely to be catheterized (27.3% vs. 10.9%, p < 0.001).
DISCUSSION
Our findings confirm that urinary symptoms are highly prevalent among patients with MSA. We found differences in the type of urinary symptoms endorsed by male and female patients, likely a reflection of their underlying biological differences. We also found differences in PVR volumes between the sexes, likely suggesting biological differences. We found intriguing differences in referral and management patterns for urinary symptoms between sexes, which appeared to influence catheterization rates.
Previous research from our group identified a higher catheterization rate in male patients with MSA [9]. We found that this difference is driven by patients who do not undergo measurement of PVR, a group in which males are twice as likely to be catheterized than females. Alternatively, when patients with MSA are referred for PVR measurement, there is no sex difference in catheterization rates: as would be expected, they are similarly low when PVR is not elevated and higher when it is. This is in line with recommendations for medical therapy in patients without elevated PVR [11] and clean intermittent catheterization for those with elevated PVR (> 100) [12]. Thus, as they do not influence catheterization rates in a sex-based manner, urodynamic studies could provide a standardized evaluation of lower urinary tract dysfunction.
Our findings show that males are more likely to endorse hesitancy and incomplete emptying and have a higher PVR than females. Previous studies in MSA have suggested detrusor overactivity, acontractile detrusor, intrinsic sphincter deficiency, and detrusor-sphincter dyssynergia as causes of urinary dysfunction [13], and perhaps anatomical sex differences that impact pathophysiology.
Incontinence is prevalent in elderly individuals, affecting an estimated 45%–78% of female versus 36%–75% of male nursing home residents with a mean age of 80–85 years. Unfortunately, systematic reviews have not been successful in identifying a sex difference in urinary incontinence in the general population due to broad definitions of the condition [14]. Although incontinence, a severe symptom on the spectrum of urinary dysfunction, was reported more frequently by the female patients in our cohort, there was no difference between males and females in referral rates for measurement of PVR. There are significant consequences for untreated urinary incontinence. Despite the degree to which it is underreported and undertreated, incontinence is associated with poor subjective quality of life [15]. Weekly episodes of urinary urge incontinence in the elderly general population significantly increase the risk of falls and nonspinal fractures, risks which are already elevated in people with prominent motor dysfunction such as MSA patients [16]. Despite this, it is estimated that just half of female patients seek care for their urinary incontinence, suggesting minimization of this symptom by patients and providers alike, and reinforcing the importance of inquiring about urinary symptoms in patients with MSA, regardless of sex [17-19].
Our large, single-center cohort of MSA patients is a strength that permits evaluation of sex differences in urinary dysfunction and its management. One limitation is that most patients did not have long-term follow-up, yet analysis of both short- and long-term patients allows us to evaluate practice patterns between those seen on a consultative basis versus those followed through their disease process. Our study cohort includes a significantly higher proportion of catheterized patients who were followed for a longer period. As such, our study has revealed that as the disease progresses, urinary symptoms may increase, requiring changes in management [12]. There was no difference in time from urinary symptom onset to catheterization between groups, suggesting that our practice is similar to referring providers. Additionally, the difference in the percentage of males compared to females who are already catheterized at presentation to our center suggests that sex differences in treatment are also prominent at other centers. However, this should be evaluated further with a multicenter approach.
Another limitation that must be considered is the absence of an age-matched healthy control cohort for comparison. Urinary symptoms are prevalent in the aging population, and the etiology may be multifactorial, including BPH in males and pelvic floor dysfunction in females. However, the significant failure rates of interventions directed at these suspected etiologies implicates alternative pathology. Furthermore, regardless of the role of sex in urinary symptoms in the normal aging population, the fact that females were referred less than males relative to the frequency of severe symptoms implies that we approach urinary symptoms differently depending on whether the patient presents as male or female and should prompt further consideration.
The absence of self-identified gender in our cohort limits the application of these findings to the diversity of gender identities but does provide an opportunity to further characterize the effect of gender identity on urinary symptoms in neurodegenerative diseases in the future. However, the fundamental differential in allocation of care implicitly invokes societal understanding of sex differences, and thus, the concept of gender cannot be isolated from consideration of this topic.
In summary, our findings detail differences that seem to be driven by biological sex (PVR volumes), societal influences of gender (referral for PVR measurement), and the relationship between the two (types of urinary symptoms that are reported), all of which ultimately influence diagnosis and management.
Notes
Data Sharing
The anonymized dataset will be shared with any qualified investigator upon request.
Conflicts of Interest
All authors have no financial disclosures or conflicts of interest for the preceding 12 months.
Funding Statement
This work was supported by CTSA Grant Number UL1 TR002377, Dominium Foundation Career Development Award in Neurodegenerative Disease Research in memory of Jack W. Safar from the National Center for Advancing Translational Science (NCATS), NIH (R01 NS092625, UL1 TR000135), Michael J. Fox Foundation, Bishop Dr. Karl Golser Foundation, Sturm Foundation, Mayo Center for Regenerative Medicine, and Mayo Funds.
Author Contributions
Conceptualization: Elizabeth A. Coon. Data curation: Elizabeth A. Coon. Formal analysis: Elke Schipani Bailey, Elizabeth A. Coon. Funding acquisition: Elizabeth A. Coon. Investigation: Elizabeth A. Coon. Methodology: Elke Schipani Bailey, Elizabeth A. Coon. Project administration: Elizabeth A. Coon. Supervision: Elizabeth A. Coon. Validation: Elke Schipani Bailey, Sara J. Hooshmand, Elizabeth A. Coon. Visualization: Elke Schipani Bailey, Elizabeth A. Coon. Writing—original draft: Elke Schipani Bailey. Writing—review & editing: all authors.