Constipation is Associated With Mild Cognitive Impairment in Patients With de novo Parkinson’s Disease
Article information
Abstract
Objective
The association between gastrointestinal (GI) symptoms and cognitive profile in patients with Parkinson’s disease (PD) at diagnosis remains unclear, although GI symptoms and cognitive impairment are highly prevalent in patients with PD. We investigated the relationship between constipation and cognitive status. We also aimed to identify the correlation between constipation and each neuropsychological dysfunction.
Methods
A total of 427 patients with de novo Parkinson’s disease with normal cognition (PD-NC, n = 170) and Parkinson’s disease with mild cognitive impairment (PD-MCI, n = 257) at Korea University Guro Hospital in Seoul, Korea were included. All patients underwent comprehensive neuropsychological tests and completed the Non-Motor Symptoms Scale (NMSS). The frequency and severity of constipation were assessed using the NMSS GI symptoms scale, we used logistic regression analysis and partial correlation analysis to determine the associations between constipation score, MCI, and each neuropsychological dysfunction.
Results
Frequent and severe constipation was associated with MCI in patients with PD at diagnosis regardless of disease severity. Specifically, constipation was related to poor performance in frontal-executive and visuospatial functions after controlling for age and sex.
Conclusion
Our findings may provide an understanding of constipation as a marker associated with cognitive impairment in individuals with PD. Therefore, the evaluation of cognitive function is warranted in PD patients with constipation, while further studies are necessary to investigate the detailed mechanism of our results.
Parkinson’s disease (PD) is a common degenerative disorder characterized by motor deficits. Patients with PD also have a variety of nonmotor symptoms, including cognitive impairment. The incidence of mild cognitive impairment (MCI) in PD patients has been revealed to be approximately 40% to 60% at diagnosis [1]. Patients with PD with MCI (PD-MCI) are more likely to be diagnosed with dementia [2], which in turn leads to poor prognosis. Thus, early detection of and intervention for MCI in PD patients is important to prevent the conversion to dementia.
Gastrointestinal (GI) symptoms are highly prevalent in PD, and constipation has been regarded as a prodromal feature, even before the onset of motor deficits. In addition, sialorrhea and dysphagia are also common symptoms that interactively affect one another and are associated with poor outcomes in PD patients [3]. Many pathology studies have revealed that alpha-synuclein deposition was observed in GI tissues more than 20 years before the diagnosis of PD, which suggests the pathogenesis of the brain-gut axis in PD. Recently, one study suggested that constipation was a predictor of cognitive decline in PD [4], and another study showed that the gut microbiota of patients with PD-MCI was different from that of patients with PD with normal cognition (PD-NC) [5].
Although there is growing evidence that pathology of the GI tract is closely related to the progression of PD, the association between GI symptoms, especially constipation, and the cognitive profile of patients with PD at diagnosis remains unclear. Therefore, the first aim of our study was to investigate the association between the presence of constipation and cognitive status. The second aim was to explore the relationship between scores of constipation, sialorrhea or dysphagia and cognitive status. Finally, we aimed to evaluate the correlation between the constipation score and each neuropsychological dysfunction.
MATERIALS & METHODS
Study participants
We retrospectively enrolled 427 patients with de novo PD (170 patients with PD-NC and 257 patients with PD-MCI) at the movement disorder clinic of Korea University Guro Hospital in Seoul, Korea, from January 2016 to December 2020. All patients underwent comprehensive movement disorder evaluation, including the Unified Parkinson’s Disease Rating Scale (UPDRS) [6], the Hoehn and Yahr scale, Non-Motor Symptoms Scale (NMSS) [7], brain magnetic resonance imaging and a standardized neuropsychological test (Seoul Neuropsychological Screening Battery 2nd edition, SNSB-II [8]). We excluded patients who had any of the following conditions: 1) drug-induced parkinsonism; 2) history of treatment for PD in other hospitals; 3) severe white matter hyperintensities (WMHs) [9], which were defined as deep WMHs ≥ 25 mm and periventricular WMHs ≥ 10 mm on fluid attenuated inversion recovery images; and 4) territorial infarction, lobar hemorrhage, brain tumor, hydrocephalus or other structural lesions.
All patients with PD-NC fulfilled the following criteria: 1) were diagnosed with PD based on the UK Brain Bank criteria [10]; 2) had no objective cognitive impairment or objective cognitive impairment in only one neuropsychological test; 3) had no medical history that was likely to affect cognitive function based on Christensen’s health screening criteria [11]; and 4) had no significant impairment in activities of daily living. All patients with PD-MCI fulfilled the movement disorder society (MDS) level II criteria for PD-MCI: an objective cognitive impairment below -1.0 SD on at least two neuropsychological tests, in which either two impaired tests were in one cognitive domain or one impaired test was in two different cognitive domains [12].
The Institutional Review Board at Korea University Guro Hospital approved this study (IRB no. 2012GR0099). Written informed consent was obtained from the patients.
Assessment of GI symptoms
Patients reported the presence of constipation, defined as bowel action less than three times weekly, and completed the NMSS, which is a well-validated questionnaire for the assessment of nonmotor symptoms [7]. The scores on the NMSS ranged from 0 to 360, with higher scores implying a higher severity and frequency of nonmotor symptoms. Scores in items assessing GI symptoms, including sialorrhea, dysphagia and constipation, were used separately in the analyses.
Assessment of cognitive function
All patients underwent neuropsychological testing using the SNSB-II [8]. We chose to use ten cognitive measures, which were representative and important neuropsychological tests, to evaluate cognitive function in five cognitive domains as follows: 1) Memory: the Seoul Verbal Learning Test (SVLT) delayed recall (verbal memory) and Rey-Osterrieth Complex Figure Test (RCFT) delayed recall (visual memory); 2) Language: the Korean version of the Boston Naming Test (K-BNT) and the animal component of the Controlled Oral Word Association Test (COWAT); 3) Visuospatial function: the RCFT copying test and clock drawing test; 4) Frontal executive function: the phonemic component of the COWAT and the Stroop Test (color reading); and 5) Attention: the Digit Span Test backwards and the Stroop Test (word reading). The results with numeric continuous values were converted to z-scores using the age, sex and education criteria presented in the SNSB-II, and then z-scores were used in the analysis.
Statistical analyses
We used analysis of variance and chi-square tests to compare the demographic data, motor scale scores, the score of each GI symptom and neuropsychological test scores between the PDNC and PD-MCI groups. To determine the association between the score of each GI symptom and cognitive impairment, we used multivariate logistic regression analysis, including the presence of constipation, sialorrhea score, dysphagia score or constipation score as independent variables, after controlling for age, sex, years of education, presence of hypertension and diabetes, disease duration and the UPDRS part III score. To evaluate the relationship between constipation and each neuropsychological test, we used partial correlation analysis after controlling for age, sex, years of education, presence of hypertension and diabetes, disease duration and the UPDRS part III score.
All reported p-values were two-sided, and the significance level was set at 0.05 in the logistic regression analysis. All analyses were performed using R version 3.6.1 (Institute for Statistics and Mathematics, Vienna, Austria; https://www.r-project.org).
RESULTS
Clinical characteristics of study patients
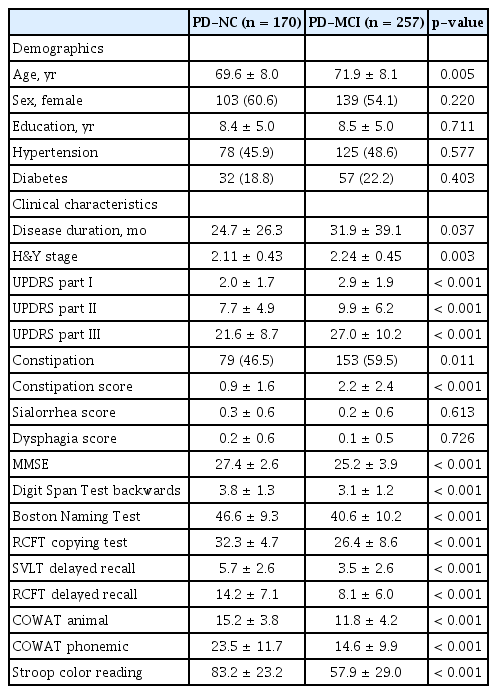
Among 427 patients with PD, there were 170 individuals with PD-NC and 257 individuals with PD-MCI. Patients with PD-MCI were older (p = 0.005) and had higher UPDRS part III (p < 0.001), frequency of constipation (p = 0.011) and constipation scores (p < 0.001) than patients with PD-NC (Table 1). The ratio of females (p = 0.220), years of education (p = 0.711), frequency of hypertension (p = 0.557) and diabetes (p = 0.403), sialorrhea score (p = 0.613), and dysphagia score (p = 0.726) were not different between the PD-NC and PD-MCI groups (Table 1).
The association between GI symptoms and cognitive impairment
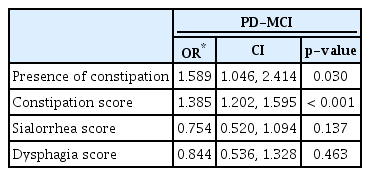
Table 2 shows the results of multivariate logistic regression analyses for independent clinical factors for patients with PD-MCI. The presence of constipation (odds ratio [OR] = 1.589, 95% confidence interval [CI] 1.046 to 2.414) or a higher constipation score (OR=1.385, 95% CI 1.202 to 1.595) was associated with cognitive impairment in PD after controlling for age, sex, years of education, presence of hypertension and diabetes, disease duration, and the UPDRS part III score. However, sialorrhea or dysphagia scores were not associated with cognitive impairment in PD.
The relationship between constipation score and performance on neuropsychological tests
We investigated the relationship between the constipation score and score in each neuropsychological test (Table 3 and Figure 1). The results showed that the constipation score was negatively correlated with scores in the phonemic component of the COWAT (p = 0.002) and color reading component of the Stroop Test (p = 0.001). However, the constipation score did not correlate with scores on the RCFT copying test (p = 0.012), SVLT delayed recall (p = 0.053), RCFT delayed recall (p = 0.034), K-BNT (p = 0.039), animal component of the COWAT (p = 0.127) and Digit Span Test backwards (p = 0.895).

The correlations between constipation score and scores in neuropsychological tests. A: Values depicted in the scatter plot represent constipation score on X-axis and phonemic COWAT score on Y-axis. B: Values depicted in the scatter plot represent constipation score on X-axis and Stroop test score on Y-axis. COWAT, Controlled Oral Word Association Test.
DISCUSSION
We investigated the relationship between GI symptoms and cognitive function in non-demented individuals with de novo PD. The major findings in this study were as follows: 1) frequent and severe constipation was associated with cognitive impairment in patients with PD at diagnosis; and 2) frequent and severe constipation was associated with poor performance in frontal-executive function. Taken together, our findings may provide an understanding of constipation as a marker associated with cognitive impairment in individuals with PD.
Our first finding was that frequent and severe constipation was associated with cognitive impairment in patients with PD, regardless of disease severity. Although there have been no comprehensive studies that explain our findings, several possible mechanisms have been proposed. First, the disruption of gut microbiota diversity caused by alpha-synuclein pathology might be associated with constipation, which would lead to cognitive impairment in patients with PD. Recently, constipation has been regarded as a surrogate marker in gut dysbiosis [13,14], which in turn aggravates neuroinflammation and neurodegeneration. One microbiome study showed that the gut microbiota diversity of patients with PC-MCI was decreased compared with that of patients with PC-NC [5]. Furthermore, fecal metabolome changes induced by gut microbiome alterations were associated with cognitive impairment in the PD group [15]. Second, given that constipation and cognitive impairment are cholinergic syndromes in the peripheral and central nervous systems, respectively, cholinergic neural loss in the basal forebrain might be another reason for the association between constipation and cognitive impairment in patients with PD. A number of studies reported that cholinergic neurons were more severely damaged in patients with PD-dementia than in those with PD-NC [16-20]. Of note, Titova et al. [21] proposed the concept of the cholinergic subtype in PD, which is characterized by cognitive impairment and GI dysfunction.
Our second finding was that frequent and severe constipation was associated with poor performance in frontal-executive function. In line with Braak staging, cortical atrophy in frontoparietal regions was observed even in the early stage of PD, and frontoparietal atrophy was correlated with motor symptom progression and frontal executive dysfunction [22,23]. In this regard, constipation might be closely related to alpha-synuclein pathology, which is further linked to the PD-specific cognitive decline pattern. Our results were also consistent with the well-known fact that the pattern of cognitive impairment in PD-MCI has been regarded as subcortical type, which is caused by damage to the cortical-basal ganglia-thalamocortical loop [24].
Our study has several limitations. First, because of the cross-sectional design, the temporal relationship between constipation and cognitive impairment remains unclear. However, this issue was mitigated to some degree by the chronology of GI symptoms and cognitive impairment in individuals with PD. Second, we assessed constipation using only subjective questionnaires. Further study is needed to investigate the association between cognitive impairment and objective constipation measures such as colon transit time or other independent scales. Third, we could not consider medications such as prokinetics and laxatives, which might affect constipation. Fourth, we could not consider the effect of Alzheimer’s disease pathologies, including amyloid and tau, which were also associated with gut dysbiosis and cognitive impairment. Nevertheless, our study is noteworthy because we provide an understanding of constipation as an associated marker with cognitive impairment in individuals with PD at the time of diagnosis. Our findings suggest that evaluation of cognitive function is warranted in PD patients with constipation. Furthermore, early intervention, such as the use of cholinesterase inhibitors, may be considered for PD patients with concomitant constipation and cognitive impairment, while further studies should be performed to investigate the detailed mechanism of our results.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
None.
Author Contributions
Conceptualization: Sung Hoon Kang, Seong-Beom Koh. Data curation: Sung Hoon Kang, Jungyeun Lee. Formal analysis: Sung Hoon Kang. Investigation: Sung Hoon Kang. Methodology: Sung Hoon Kang. Supervision: Seong-Beom Koh. Writing—original draft: Sung Hoon Kang. Writing—review & editing: Sung Hoon Kang, Seong-Beom Koh.