Orthostatic Hypotension in Drug-Naïve Patients with Parkinson’s Disease
Article information
Abstract
Background and Purpose
Orthostatic hypotension (OH) is known to be present even in patients with early Parkinson’s disease (PD). To affirm the presence of OH and find correlation between OH and other dysautonomic symptoms in PD, this study has done in newly-diagnosed PD patients.
Methods
Forty-five non-demented patients with no prior history of treatment for PD were recruited (17 men, 63.8 ± 10.1 years of age). All the patients were evaluated for OH before starting medications. Autonomic symptoms were evaluated with structured questionnaires. Clinical characteristics of PD were evaluated (median Hoehn and Yahr stage 2.0 (1–3), 1.3 ± 1.1 years of disease duration), and comorbid medical conditions that could affect blood pressure were also recorded.
Results
OH was prevalent, and eighteen patients (40%) showed orthostatic hypotension, and twenty-seven (60%) did not (normotensive group). There was no significant difference in demographic and clinical characteristics between groups. The presence or severity of symptoms of autonomic dysfunction in the OH group also not differed from those of the normotensive group.
Conclusions
OH was prevalent even in the early stage of PD, and was not related to presence or severity of any other symptoms of autonomic dysfunction. Our findings suggest that clinicians should pay attention to OH from the early stage of disease.
Clinically, Parkinson’s disease (PD) is mainly characterized by motor symptoms such as bradykinesia, rigidity, tremor, and postural instability. However, patients with PD may also experience many complaints beyond motor symptoms, which are referred to as non-motor symptoms.1 The non-motor symptoms of PD are very diverse, including neuropsychiatric symptoms, sleep disturbances, sensory symptoms, and dysautonomic symptoms.2 It has become increasingly apparent that non-motor symptoms have a quite considerable impact on the quality of life of PD patients.1
Impaired cardiovascular response to postural changes, particularly orthostatic hypotension (OH) is considered an important clinical and even diagnostic feature for the multiple system atrophy or pure autonomic failure.3,4 In PD, OH was thought to develop later in the disease course or only in more severe cases often in elderly patients.5–7 Moreover, OH has been thought to be a side effect of anti-parkinsonian medications, like levodopa and dopamine agonists.8–12 However, it is accepted that OH is also a common finding even in the early stages of PD.13,14
The authors evaluated OH among newly-diagnosed PD patients and the relationship between OH and other symptoms of autonomic dysfunctions in patients.
Methods
Subjects
We studied 45 selected PD patients without dementia. The subjects had been diagnosed as PD for the first time or diagnosed as PD by other clinicians but had never been treated before. The diagnosis of PD was established according to the criteria of the United Kingdom Parkinson’s Disease Society Brain Bank.15 We excluded atypical or secondary parkinsonisms, and the diagnosis was confirmed during the follow up visits for more than 6 months. Comorbid medical conditions that could affect blood pressure were also evaluated, which included hypertension, diabetes mellitus, and benign prostatic hypertrophy.
Evaluation of parkinsonian symptoms
Severity and stage of parkisonism were rated using the unified Parkinson’s disease rating scale, the Hoehn and Yahr stage and the Modified Schwab and England activities of daily living.
Evaluation of orthostatic hypotension and autonomic dysfunction
All patients were evaluated for the presence of OH, regardless of symptoms of orthostatic dizziness or syncope. To avoid the blood pressure lowering effect of dopaminergic drugs, all patients evaluated for OH before medication. OH was measured by monitoring systolic and diastolic blood pressure and heart rate. After a 10 minute period of lying down, systolic and diastolic blood pressures were checked in the first, third, and fifth minutes after standing up. If the patient could not stand up by his or herself, a tilt table was used. OH was defined as a drop in systolic BP ≥ 20 mmHg or diastolic BP ≥ 10 mmHg at anytime following standing up.
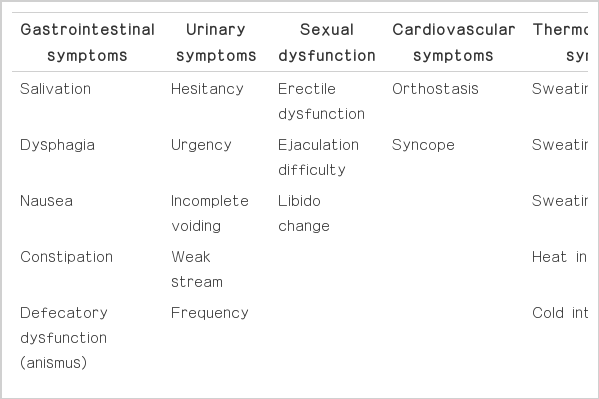
Comprehensive assessment of autonomic dysfunction was also assessed using a systematized autonomic dysfunction questionnaire (ADQ).3 The ADQ covered gastrointestinal symptoms, urinary symptoms, sexual dysfunction, cardiovascular symptoms and thermoregulatory symptoms. The severity or frequency of each of the symptoms was expressed as scores (Table 1).
Statistical Analysis
SPSS version 14 for Windows was used for statistical calculations. Mann-Whitney and Chi tests were employed to analyze intergroup difference. Statistical significance was declared at the p < 0.05 range.
Results
General and clinical characteristics of patients
The subjects (17 men and 28 women, age of 63.8 ± 10.1 years) showed mild disease [median Hoehn and Yahr stage 2.0 (1–3)] with 1.3 ± 1.1 years of disease duration. Twenty-two patients had comorbid disease, which could affect blood pressure. Hypertension (20/45 patients) was the most common, followed by diabetes (7/45) and benign prostatic hyperplasia (1/45).
Eighteen patients showed OH (OH group, 8 men, age 61.3 ± 10.8 years) and twenty-seven patients (normotensive group, 9 men, age 65.5 ± 9.4 years) did not. Patients without OH were older and showed shorter duration of education, but those demographic characteristics were not significantly different between groups (Table 2). Parkinsonian symptoms and daily activities of the patients also showed no significant difference between groups (Table 2).
Symptoms of autonomic dysfunctions in patients
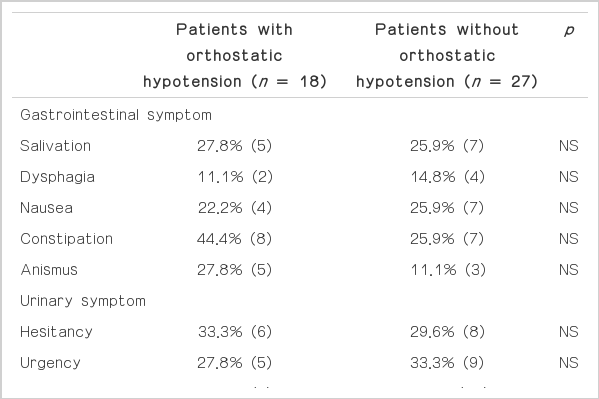
Many dysautonomic symptoms were accompanied with OH in patients, and all the patients had more than two of those symptoms (Table 3). Urinary symptoms were most common and followed by sexual symptoms. Although urinary incontinence was more prevalence in OH group, it did not reach statistical significance. The presence of OH was not related with the presence of other symptoms.
Total score in the ADQ was slightly increased in patients with OH compared to that of patients without OH (Table 4). However, the differences did not reach statistical significance. In spite of the insignificant difference, the scores of all domains in the OH group were higher than those of the normotensive group. However, even in the questions of cardiovascular symptoms, orthostatic dizziness and history of syncope, there was no difference between groups. Only eight patients complained of orthostatic dizziness and four of those patients showed OH.
Discussion
Abnormal cardiovascular regulation, including orthostatic hypotension was frequently expressed in various stages of PD patients.16,17 The cardiovascular autonomic disturbances are following the gastrointestinal symptoms in frequency, but they have a higher impact on the quality of life in PD patients.
Lewy bodies, a pathological hallmark of Parkinson’s disease, have been found in autonomic regulatory regions of the brain or peripheral autonomic ganglia in PD patients.18 And, many studies have shown the evidence of cardiac sympathetic denervation in early PD patients by using cardiac sympathetic imaging.19,20
Symptoms of autonomic dysfunction in PD patients were known to be related with older age, long disease duration, severe disease stage and anti-parkinsonian medication.7 However, Allcock et al. showed that frequency of OH was independent of duration and severity of movement symptoms in the study of the frequency of OH in a community-based cohort of PD patients.13 The prevalence of OH in PD was variable according to criteria and methods. In 5 relatively large studies involving more than 80 patients each, the frequency of OH ranged from 30% to 58%.6 Bonuccelli et al.21 showed the high prevalence of OH in de novo PD patients. In our study, prevalence of OH in the drug-naïve PD patients was moderate (18/45 patients, 40%), and similarly shown in the patients without comorbid disease (9/23 patient, 39.1%). Patients with OH were young and showed a short duration of disease, but those differences did not reach statistical significance, and the severity of parkinsonian symptoms was similar to those of patients without OH. The presence or severity of dysautonomic symptoms was not different between patients with or without OH, even in the cardiovascular domain. Therefore, OH did not respect the severity or distribution of other autonomic symptoms. Actually, the complaint of orthostasis was made by only six patients (13.3%), four of those patients showed OH. Most patients with OH did not complain of orthostasis, and Steven et al. showed that the majority of patients with profound orthostatic hypotension either did not show the typical symptoms (33%), or showed only atypical symptoms (24%).22 The unawareness of orthostatic hypotension stresses the importance of blood pressure monitoring irrelevant to complaints of patients.
Although we could not find statistical significance, patients with urinary incontinence were much higher in OH group (27.8% vs. 7.4%). This finding suggests that those patients can be diagnosed as multiple system atrophy (MSA). We followed all the patients more than six months and confirmed the good response to dopaminergic treatment. Because some patients with MSA show good response to treatment in early period, we could not exclude the possibility of MSA. Further studies with longer period of observation and about the change of dysautonomic symptoms along the disease course are warranted.
Although pathological implications of autonomic dysfunctions in PD patients have not been established yet, clinical implications can be significant enough to impact the daily activities of PD patients. Furthermore, most patients do not complain of noticeable symptoms, and the unawareness of patients warrants careful evaluation. Appropriate management of OH can prevent further insult and can improve daily activities of PD patients.
Notes
The author has no financial conflicts of interest.