Acquired Movement Disorders Secondary to Giant Tumefactive Perivascular Spaces
Article information
Dear Editor,
Perivascular spaces (PVSs), commonly known as Virchow-Robins spaces, are interstitial cystic spaces filled with cerebrospinal fluid (CSF). PVSs generally accompany deep branches of cerebral vessels within the white matter and, in healthy individuals, are < 2 mm in diameter [1]. Traditionally, these spaces are asymptomatic and benign; however, in rare instances, these spaces can expand into giant tumefactive PVSs (GTPVSs) that compress nearby structures, causing neurologic disorders and hydrocephalus [1,2]. PVSs > 15 mm in diameter are considered GTPVSs and may require neurosurgical intervention for symptomatic relief [1,3].
A 17-year-old man with a history of congenital hypothyroidism presented with chronic, nonprogressive left upper extremity shaking and dysconjugate gaze with compensatory tilt since the age of 11 years. On examination, he had vertical nystagmus, saccadic upward gaze, left hyperopia with skew deviation and trace bilateral papilledema. Initial MRI revealed a 4 cm multicystic lesion in the left midbrain that extended into the thalamus and partially obstructed the third ventricle, causing dilatation of the third ventricle with mild lateral ventricular dilatation (Figure 1A and B). At the age of 12 years, he underwent open biopsy with soft tissue at the superior portion of the brainstem and cystic fenestration. Pathology showed minute fragments of gray and white matter without neoplastic cells (Figure 1E and F). He has yearly MRI scans of the brain that show stable ventricular size and no evolution of GTPVSs. Because his symptoms do not bother daily living activities, the decision was to clinically and radiologically monitor (Figure 1C and D).
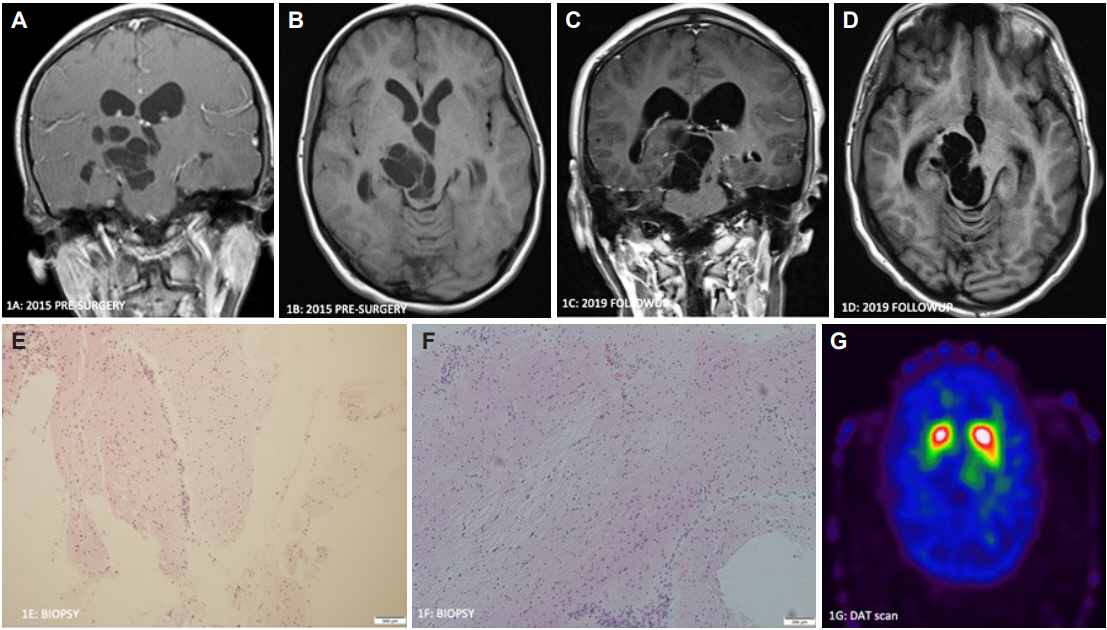
Coronal T1 postcontrast (A) and axial T1 sequence (B) (age 12 years old, presurgical) demonstrate a large, right-sided cystic mesencephlothalamic lesion and dilated lateral ventricles. Coronal T1 postcontrast (C) and axial T1 (D) (age 16 years old) demonstrate a stable lesion. Biopsy specimen shows normal white and gray matter (H&E, magnification ×100) (E and F). DAT scan (age 17 years old) demonstrates decreased uptake in the right putamen and normal uptake in the left striatum (G).
Physical examination (at the age of 17 years) revealed left head tilt and mild scanning speech. The left eye had an elevated gaze compared to the right eye with skewed lateral left and right gaze as well as intermittent horizontal and rotatory nystagmus. He had a 4–6 Hz proximal postural tremor in the left upper extremity with dystonic posturing of the left hand. Handwriting with the left hand was jerky, ataxic and required purposeful force to keep the pen stable on the paper. When asked to pour water from a cup in one hand to the other, the left-sided tremor worsened. Finger-to-nose testing showed ataxia without dysmetria; however, when he used his right hand to stabilize the left arm, finger-to-nose testing showed a marked improvement in ataxia. Mild left-sided bradykinesia was noted, but tone was normal. There was no postural instability. Ataxia was also noted with heel-to-shin testing on the left and tandem gait (Supplementary Video 1 in the online-only Data Supplement).
A DATscan [preprocedure intravenous infusion of I-123 ioflupane as well as SPECT and CT images of the brain were acquired and processed using Symbia Intevo 16 SPECT/CT hybrid technology (Siemens Healthineers, Malvern, PA, USA)], which revealed normal uptake in the left striatum, decreased uptake in the right putamen and mild decreased uptake in the right caudate nucleus (Figure 1G). The levodopa trial did not help the tremor.
We present a rare case of an acquired movement disorder in a pediatric patient secondary to GTPVSs. Our patient’s complex movement disorder and abnormal extraocular movements correspond to the location of his GTPVSs. It has been postulated that these expanding PVSs are due to defects in drainage of interstitial fluid into the ventricular system and not the result of increased intraventricular pressure. In every case reported in the literature, mesencephalo-thalamic expanding lacunae are associated with hydrocephalus. We believe hydrocephalus was the consequence of compression of the aqueduct by the lacunae rather than its cause. Impingement on right cerebellothalamic fibers is the likely etiology for left upper extremity proximal tremor and left-sided ataxia. Abnormal DaT scans are likely a result of compression of dopaminergic presynaptic neurons. The lesion likely compressed the cerebellothalamic fibers and right thalamus, which may have resulted in impaired eye motility. Tumefactive PVSs with mass effects and obstructive hydrocephalus can be treated surgically with ventriculostomy, cyst fenestration, ventriculoperitoneal shunting or cystoperitoneal shunting. Al Abdulsalam et al. [1] described a case of GTPVSs that collapsed following the insertion of a ventriculoperitoneal shunt. In most of the patients, the surgical procedure ameliorated gait difficulties, bradykinesia, urinary urgency and sudden falls, while other signs, such as tremor or restriction of the upward gaze, were not modified. Our patient did not require shunt placement as his symptomatology did not interrupt activities of daily living and stable lesion size over 5 years. He will continue to be monitored closely with serial imaging.
Another important aspect when managing patients with brain cystic lesions is to appropriately diagnose the lesions. The CSF like signal intensity without contrast enhancement or perilesional edema in the cystic lesion of our patient is consistent with the diagnosis of giant VRSs. The main differential diagnoses of giant VRSs on MRI are cryptococcosis, mucopolysaccharidoses, cystic neoplasms, colloid cysts, arachnoid cysts, dermoid cysts, epidermoid cysts, and neurocysticercosis [2].
The predominant movement disorder that results from dilated PVSs is parkinsonism (predominantly asymmetric) with a variable response to levodopa [4,5]. Other neurological manifestations that have been reported are focal dystonia, dementia and hydrocephalus. The most common presenting symptom for GTPVSs is headache. Our case is unique given the first description of proximal postural tremor as a presenting symptom.
This case highlights that dilated PVSs are not always benign entities and can result in complex neurologic presentations. Virchow-Robin space enlargement should be considered part of the differential diagnosis in patients with cysts mainly located in the mesencephalon.
The implications, pathogenesis and proper management of such lesions require further research.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.20112.
Video 1.
Video 1. Young man with left upper extremity tremor who presents with posture and action only. In the initial part of the video, he describes how the tremor can be controlled if he supports his left upper extremity. Left-hand dystonia is also noted, which worsens when he performs a task with the right hand. He has difficulty holding a pen with the left hand because of impaired coordination and dystonia. The left upper extremity action tremor worsens when pouring water from one cup to another, and the tremor is predominantly proximal. He also has impaired tandem gait, and his left shoulder is pulled down.
Notes
Ethics Statement
The authors confirm that the approval of an institutional review board was not required for this work. Informed written consent was obtained, and patient consent was shared.
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Zain Guduru. Supervision: David Neil Toupin, Donita Lightner, Zain Guduru. Writing—original draft: Anthony J. Donigian, David Neil Toupin. Writing—review & editing: Donita Lightner, Zain Guduru.
Acknowledgements
None
