Chorea as a Presentation of SARS-CoV-2 Encephalitis: A Clinical Case Report
Article information
Central nervous system (CNS) involvement in SARS-CoV-2 is now a known fact, likely due to viral transmission through the olfactory nerve and high brainstem viral load, which also suggests dissemination in the ambiguus and solitary nuclei from the respiratory tract via the vagus nerve [1]. Anosmia and ageusia findings are justified by a potential large cohort study. It may only be an interesting coincidence that hyposmia disorders are normal in premature Parkinson’s disease (usually only in the prodromal phase) and the olfactory system is an early favored site for alpha-synuclein linked diseases [2].
Chorea is a hyperkinetic movement disorder characterized by involuntary brief, sudden and irregular movements [3]. Chorea patients may not recognize irregular movements immediately since they are discreet, and some gestures can be temporarily blocked by parakinesia. The inability to maintain voluntary contraction, as observed during the milkmaid grip test and in individuals with persistent tongue protrusion, is a characteristic of chorea. Chorea can cause irritability and gaucheness. Chorea can be hereditary or acquired, generalized or localized and can have several etiologies, such as viral infection and acute stroke.
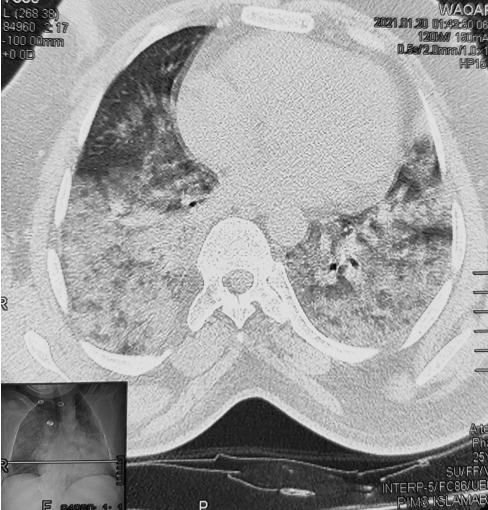
A 58-year-old male, with known hypertension and no travel history, was brought to the emergency room (ER) at 1:00 pm with complaints of abnormal movements of the hands and feet with an inability to talk properly. The patient was very irritable during the initial ER visit, and intravenous (IV) midazolam was given to control movements. Consultation with the neurology department was requested (Supplementary Video 1 in the online-only Data Supplement). The neurology resident on duty received the patient with a blood pressure of 120/80 mm Hg, a respiratory rate of 30 breaths/min, a pulse of 110/min and a temperature of 38.9°C, along with an inability to complete a single sentence. He had bilateral coarse crepitation with mild wheezing. His neurological examination showed a Glasgow Coma Scale (GCS) of E4M6V4 with movement in all four limbs, bilateral downward plantar reflex in the flexors and pupils bilaterally equal and reactive to light. There was no loss of the sensations of smell or taste. Motor and sensory examination was limited due to persistent abnormal movement. The resident assessed him, and with the provisional diagnosis of acute chorea, he planned his CT brain scan on the basis of the baseline workup. He was assisted by a consultant neurologist at baseline, and chest X-ray was reported as showing a bilateral ground glass appearance with opacities in the right lower and middle lobes of the lung (highly suspicious of COVID-19) (Figure 1). A nasopharyngeal swab was performed, as well as cerebrospinal fluid (CSF) analysis, with proper protective equipment, and the patient was transferred to the suspected COVID-19 patient bay in the ward. According to the World Health Organization guidelines, nasopharyngeal swabs were obtained in separate sterile tubes filled with 0.5 mL phosphate-buffered saline and supplemented with 0.5% bovine serum albumin for diagnostic analysis for SARS-CoV-2. Empirical treatment of viral encephalitis was started, and the SARS-CoV-2 polymerase chain reaction (PCR) results were positive. The patient was transferred to the main COVID-19 isolation ward. His initial blood investigation showed an increased total leukocyte count with neutrophilic dominance and lymphocytopenia and increased C-reactive protein, D-dimer and serum ferritin. Subsequent investigations included systemic brain MRI, which demonstrated no gross lesions except mild periventricular ischemic changes (Fazeka-II grade) (Supplementary Figure 1 in the online-only Data Supplement). High-resolution CT showed a bilateral ground glass appearance and opacities in the right lower, middle and upper lobes of the lung (Figure 1). Further CSF examination showed a clear and colorless CSF with a cell count of 4/µL and no red blood cells. His CSF HSV PCR, autoimmune profile and oligoclonal bands (OCBs) were negative, but SARS-CoV-2 was detected in the CSF. After admission to the isolation ward, his GCS was E4M6V5, with a Mini-Mental State Examination and Montreal Cognitive Assessment score of 29/30 and 28/30, respectively, and he was started on IV ceftriaxone, azithromycin, acyclovir, methylprednisolone (40 mg twice daily for 7 days), omeprazole and prophylaxis for deep venous thrombosis. The patient autoimmune profile and OCBs showed negative results. He was also started on oral umifenovir, procyclidine, risperidone and amantadine sulfate. The patient improved daily in terms of oxygen saturation, encephalopathy and abnormal choreiform movement (Supplementary Video 2 and 3 in the online-only Data Supplement). Later, risperidone was discontinued, and low-dose haloperidol was added to the treatment regimen. The patient was discharged from isolation after 14 days with a GCS of E4M6V5.
Supplementary Video 2–5 (in the online-only Data Supplement) were at different times and on different days during isolation.
This case shows the neuroinvasive potential of SARS-CoV-2 and that we cannot exclude neuroinvasive involvement of SARS-CoV-2 infections if a PCR test for SARS-CoV-2 using a patient’s nasopharyngeal specimen is positive. The potential ability of SARS-CoV-2 to invade and reside within neural tissue directly or through an immune-mediated response is still controversial [4]. Recently, a study was published that showed that the most characteristic SARS-CoV-2 induced CNS central nervous system symptoms include altered levels of consciousness, delirious behavior, headaches and vertigo [5].
The most likely hypothesis proposed is frequent involvement of smell and taste fibers providing a route to the brain. The literature has supported the concept of CNS involvement secondary to respiratory tract infections through the release of damaging cytokines/chemokines released during the inflammatory process hampering and crossing the blood brain barrier. This results in an inflammation cascade inside the brain [5].
Another theory proposes the involvement of molecular-based mechanisms through which the SARS-CoV-2 virus produces antibodies against neural tissues and glial cells; this is the same mechanism by which Epstein-Barr viruses, HSV-1 and Japanese encephalitis virus work [6].
This case is of particular interest because our patient had severe neurological symptoms, including encephalitis, severe respiratory symptoms and uncontrolled acute choreiform movements. The initial presentation of the patient with signs of choreiform movements suggested neuroinvasive potential; hence, antibiotics and antiviral therapy were initiated. However, in our case, SARS-CoV-2 was detectable in CSF, which indicates the potential brain invasion of the virus. Hyperviscosity in the basal ganglia is the recurrent mechanism of chorea development, but few patients develop chorea despite signs of hyperviscosity in other organs, and patients with chorea may not have a high hematocrit. The resulting pro-inflammatory condition in the striatum may, on a theoretical level, result in neuronal dysfunction and chorea generation [7].
It is particularly important to mention that all the alterations in cytokines occurred in the blood samples, while no inflammatory or infectious marker abnormalities were seen in the CSF sample. Thus, it is believed that the neurological manifestations of COVID-19 are due to CNS-specific inflammatory cascades. Together with normal serial imaging scans showing an absence of evidence of neuronal-injury, these findings suggest the induction of altered functioning rather than any destructive mechanism of SARS-CoV-2 in the central nervous system.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.20098.
Video 1.
Patients with uncontrolled involuntary movements in ER.
Video 2, 3, 4, 5.
These videos showed patients with relatively improved uncontrolled involuntary and abrupt movements of all limbs.
MRI brain FLAIR image showed normal findings.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Muhammad Hassan, Fibhaa Syed. Data curation: Muhammad Hassan, Waleed Shahzad. Investigation: Haris Majid Rajput, Liaqat Ali, Farhan Faisal. Supervision: Mazhar Badshah. Writing—original draft: Muhammad Hassan, Haris Majid Rajput, Mazhar Badshah, Farhan Faisal, Liaqat Ali. Writing—review & editing: Fibhaa Syed, Mazhar Badshah.
Acknowledgements
None