Asymmetric Periodic Leg Movements during Sleep after Unilateral Supratentorial Infarction: Two Legs with One Lesion
Article information
Dear Editor,
Following unilateral lesions in stroke, symptomatic periodic leg movement during sleep (PLMS) that manifests as contralateral leg movements has been reported [1,2], although its anatomical substrates and neurophysiology remain elusive. Herein, we report a case of unilateral supratentorial infarctions that possibly provoked asymmetric PLMS.
A 73-year-old man was found to have acute-onset dysarthria, left facial palsy, and left hemiparesis. He had been previously diagnosed with diabetes mellitus and hypertension. He was a smoker and drank almost daily.
Physical examination revealed left facial palsy of the central type, dysarthria, and dysphagia. His left upper and lower extremities were found to be Medical Research Council grade IV for muscle strength without any sensory abnormality. The deep tendon reflexes in both lower extremities were symmetric and hypoactive, and both plantar reflexes were flexor.
Diffusion-weighted imaging revealed an acute infarction in the posterior limb of the right internal capsule and the adjacent pallidum (Figure 1). His blood tests, including hematological and renal function profiles, were unremarkable. The hemoglobin A1c level was 6.5%.
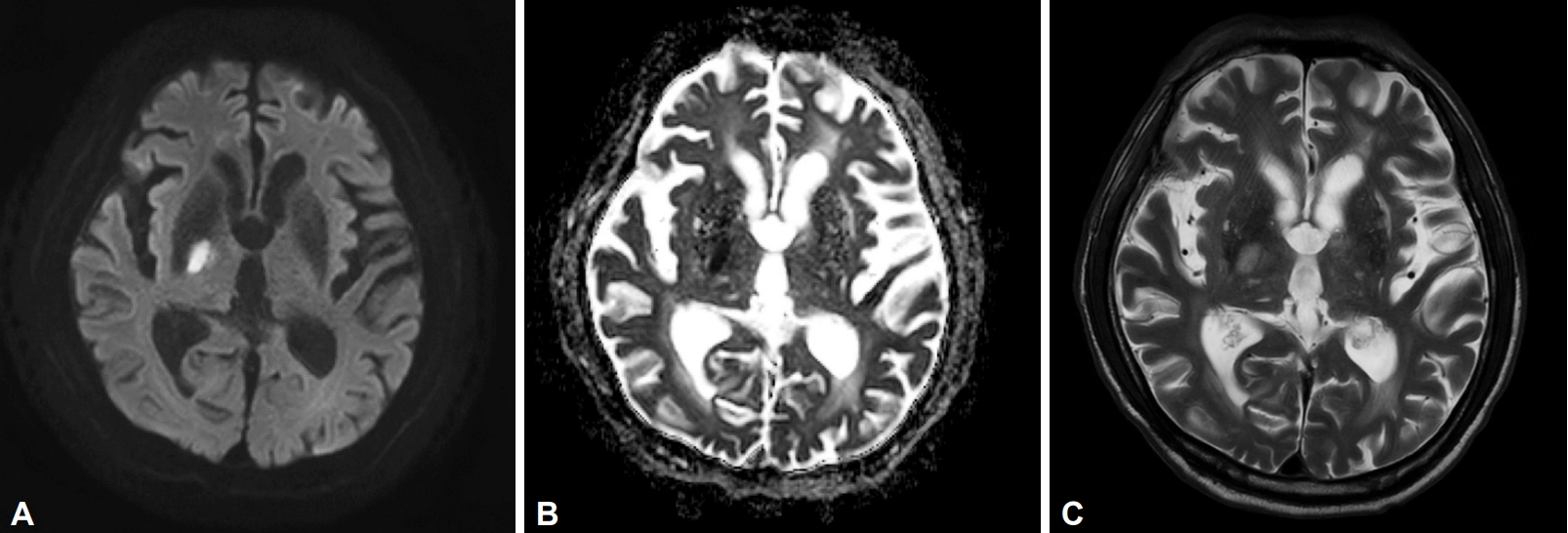
Diffusion-weighted magnetic resonance image shows (A) high signal intensity and (B) a low apparent diffusion coefficient at the posterior limb of the right internal capsule and adjacent globus pallidus, depicting acute cerebral infarction. (C) T2-weighted image also manifests high signal intensity at the corresponding location.
On the following day, the patient’s son observed an involuntary jerky movement of the patient’s left leg approximately 30 minutes after falling asleep. On scrutiny, this periodic jerky movement was composed of the extension of the great toe, ankle dorsiflexion, knee flexion, and hip flexion. We also observed jerky and mildly synchronous extension of the right big toe and ankle dorsiflexion that were usually asynchronous but rarely occurred sequentially after left leg movement. The movements were largely asymmetric, and the movements on the left side were more predominant and frequent than those on the right. Both toe movements resembled Babinski’s sign, and the magnitude of the left Babinski-like activity was greater than that of the right. These movements recurred every 20 to 35 seconds (Supplementary Video 1 in the online-only Data Supplement), they were confined to his lower extremities and they were observed in his lying-down position during light sleep.
Although we could not completely confirm his previous sleeping status, the patient’s family members did not report a history of PLMS or other sleep disorders, such as snoring or sleep apnea. In addition, the patient had not complained of any symptoms of restless leg syndrome (RLS), which is frequently comorbid with PLMS (nearly 90%). The patient did not express any distal limb paresthesia that might suggest diabetic polyneuropathy or lumbosacral radiculopathy. We did not perform overnight polysomnography; however, the abnormal motor phenomena observed during sleep were appropriate for the diagnosis of PLMS in terms of the duration of each symptom, the time interval between leg movements, and the occurrence time after sleep start. Based on his past history and sudden onset of asymmetric PLMS, with dominance corresponding to the contralateral hemispheric lesion, the patient was diagnosed with asymmetric PLMS attributable to acute unilateral supratentorial infarction.
The occurrence of PLMS with or without RLS after ischemic stroke has been infrequently reported. The corona radiata, internal capsule of the posterior limb, globus pallidus interna, and pontine areas, which incorporate extrapyramidal and pyramidal pathways, have been suggested as causative neuroanatomical substrates [1,2], and those cases involved clinical laterality crosslinked with symptomatic lesions. It was argued that a loss of cortical or subcortical inhibition by responsible destructive lesions in the brainstem reticular formation provokes the disinhibition of the reticulospinal excitatory pathway and eventually leads to contralateral PLMS [1]. Such a hypothesis also applies to our patient, where a disrupted pyramidal tract disconnected the complex circuitry of supratentorial structures from spinal generators that become integrated through the brainstem [3,4], thereby provoking PLMS.
Our case differs from previous reports in terms of its asymmetry and asynchrony. The corticospinal tract modulates related structures while descending, and it crosses just above the spinomedullary junction with asymmetry; 80% of the fibers cross contralaterally and 20% cross ipsilaterally. We can speculate that two independent, specific, spinal-pattern generators, unleashed by the disrupted integrity of the supratentorial influence, are responsible for each leg’s asynchronous activations [3,4]; the left leg expressed a triple flexion reflex and Babinski-like activity by involving 80% of the pyramidal tract, and the right leg manifested only Babinski-like motions because of the smaller involvement of the ipsilateral pathway. This disproportionate contribution of an integrated pyramidal pathway to two unrelated spinal generators resulted in different motor phenomena and asynchrony.
On the basis of the response of leg movements to dopaminergic treatments, previous reports strongly suggest that the basal ganglionic dopaminergic pathway is responsible for PLMS after stroke [2]. There are also studies that emphasize the existence of bilateral extrapyramidal pathways as well as cross-linked associations from each side. The globus pallidus projects to the contralateral thalamus and tegmentum, and corticostriatal fibers project both ipsilaterally and contralaterally [5]. A disruption of this complex loop may contribute to the involuntary movements of the patient.
In contrast, we hypothesize the importance of the pyramidal tract contribution based on the appearance of bilateral Babinski-like activities. The Babinski response is a spinal polysynaptic reflex that reliably indicates pyramidal tract diseases. Its resemblance to PLMS has been analyzed, and it was advocated that PLMS is a form of pyramidal tract expression that is disinhibited by non-rapid-eye-movement sleep suppression of the inhibitory suprasegmental influences [6]. This further strengthens our argument that pyramidal tract disruption, which contains crossed and uncrossed motor pathways, contributed to the asymmetry of symptoms seen in our patient.
Few studies report bilateral PLMS occurring after unilateral supratentorial lesions. In a prospective study of 35 patients, eight patients manifested asymmetric PLMS with dominance corresponding to the contralateral hemispheric lesion [1]. However, the authors merely assessed the occurrence and did not detail the manifestations, in particular, an emphasis on the existence of dominant laterality, as seen in our patient.
In conclusion, this case represents another occurrence of strokerelated PLMS. The pathophysiology of clinical asymmetry and asynchrony is still unclear, but mechanisms are suggested.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.20004.
Supplementary Video Legend
Video 1. During sleep, the patient manifested asynchronous and asymmetric lower-limb movements with periodicity of 20 to 30 seconds. The left leg demonstrated a triple flexion reflex with Babinski-like fanning of the toes. The right leg mainly displayed big toe and ankle dorsiflexion that did not extend to the knee and hip. The magnitude of the movements and swiftness were greater on the left side. During the movements, we did not notice snoring or an arousal of consciousness. The patients have consented to the submission of this article to the journal.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Sang-Won Yoo and Joong-Seok Kim. Investigation: all authors. Supervision: Joong-Seok Kim. Writing—original draft: Sang-Won Yoo. Writing—review & editing: Ko Eun Choi and Joong-Seok KIm.
Acknowledgements
None.
