A Case of Abnormal Postures in the Left Extremities after Pontine Hemorrhage: Dystonia or Pseudodystonia?
Article information
Abstract
It is difficult to determine the pathoanatomical correlates of dystonia because of its complex pathophysiology, and most cases with secondary dystonia are associated with basal ganglia lesions. Moreover, it is a challenging issue that patients with abnormal postures accompanied by other neurological findings in the affected body part (e.g., sensory loss) can be diagnosed with true dystonia or pseudodystonia. Here, we report a case of abnormal postures with loss of proprioception in the left extremities after right dorsal pontine hemorrhage.
Dystonia is a type of involuntary movement characterized by the patterned and repetitive contraction of agonist and antagonist muscles [1]. It can be classified into primary dystonia, dystonia-plus syndromes, heredodegenerative dystonia, and symptomatic dystonia according to its etiology [2]. In particular, focal lesions in areas such as the basal ganglia, thalamus, cerebellum and brainstem have been reported to cause symptomatic dystonia [3]. However, it is difficult to determine the pathoanatomical correlates of dystonia because of its complex pathophysiology, and most cases are associated with basal ganglia lesions [4]. Moreover, it is challenging to distinguish between dystonia and pseudodystonia [5] when abnormal postures are accompanied by other neurological findings in the affected body part. Here, we report a case of abnormal postures with decreased proprioception in the left extremities after right pontine hemorrhage.
A 47-year-old man with a history of hypertension was referred to our hospital with sudden onset of hearing loss and dizziness. Brain computed tomography showed right dorsal pontine intracranial hemorrhage extending to the lower midbrain and fourth ventricle. On the 11th day after conservative management in the intensive care unit, involuntary sustained muscle contraction developed in the left hand and foot, which worsened when standing up or stretching out the left arm. The patient had no previous or family history of movement disorders. He also had no history of exposure to relevant drugs, except for one week of antipsychotics prescribed for delirium during hospitalization.
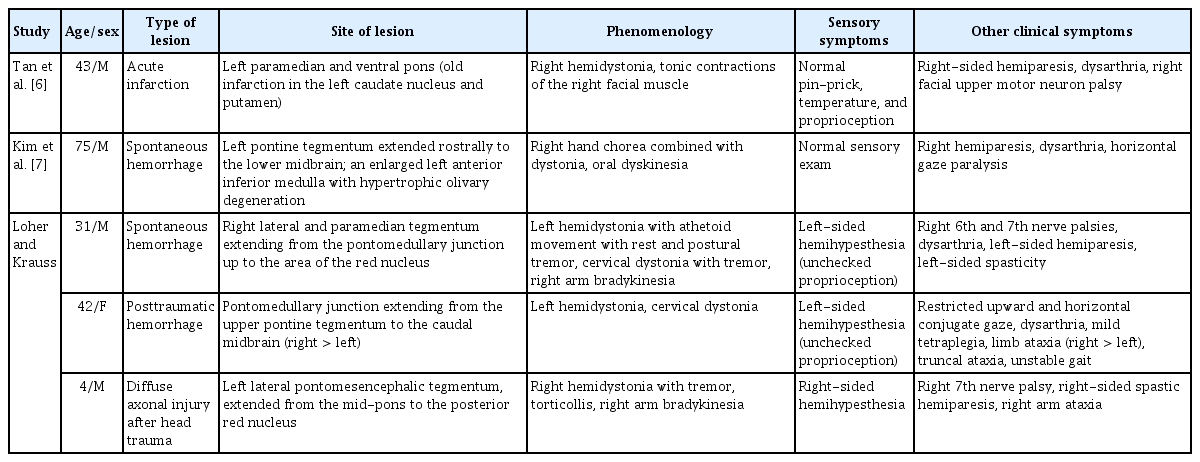
One month later, the patient was referred to the Department of Neurology for the abnormal movements of his left hand and foot. He showed extraocular muscle movement limitations, compatible with right 6th nerve palsy, and right peripheral type facial palsy with hypoesthesia. He experienced almost full improvement in hearing impairment. A motor function test demonstrated no weakness, but a sensory examination showed severe decreases in vibration and position sense of the left distal arm and leg with intact pain sensation. The Romberg test was positive, and he fell within 20 seconds of closing both eyes. Abnormal postures were observed in the left hand with patterned flexion of the wrist and hyperextension of all five fingers while stretching out his left arm, combined with fine postural tremor. Abnormal postures were also observed in his left foot with dorsiflexion of the ankle and flexion of the toes when lying down or stretching out the leg, combined with slow and irregular writhing movements. When he closed both eyes, the abnormal posture of the left hand was aggravated (Supplementary Video 1 in the online-only Data Supplement). There was no sensory tricks. Brain magnetic resonance imaging showed low signal intensity in the right dorsal pons, extending to the lower midbrain in susceptibility-weighted images. There was also a hyperintense lesion in the left pons on the T2-weighted image, indicating an old ischemic change (Figure 1A). There was no focal lesion in the cortical gray matter, basal ganglia, internal capsule, thalamus, or cerebellum. The electromyography recording showed coactivation of the left flexor carpi radialis and extensor carpi radialis while stretching out his left arm (Figure 1B) as well as coactivation of the left tibialis anterior and gastrocnemius while stretching out his left leg (Figure 1C). After the introduction of anticholinergics and clonazepam, his abnormal postures partially improved.
(A) Axial susceptibility-weighted, T1-weighted, and T2-weighted imaging 1 month later showed a hemorrhagic lesion in the right pons and a chronic ischemic lesion in the left pons. (B) The electromyography (EMG) recording showed coactivation of the left flexor carpi radialis and left extensor carpi radialis while stretching out his left arm. (C) The EMG recording showed coactivation of the left tibialis anterior and left gastrocnemius while stretching out his left leg.
Dystonia has been traditionally regarded as a disorder of the basal ganglia, but recent studies have suggested that it is a network disorder involving the cerebello-thalamo-cortical circuit [4]. In addition, some reports have shown that the brainstem can be associated with the pathogenesis of dystonia; however, structural lesions are not limited to the brainstem in most cases, and hemidystonia has rarely been reported as a consequence of focal brainstem lesions [6-8].
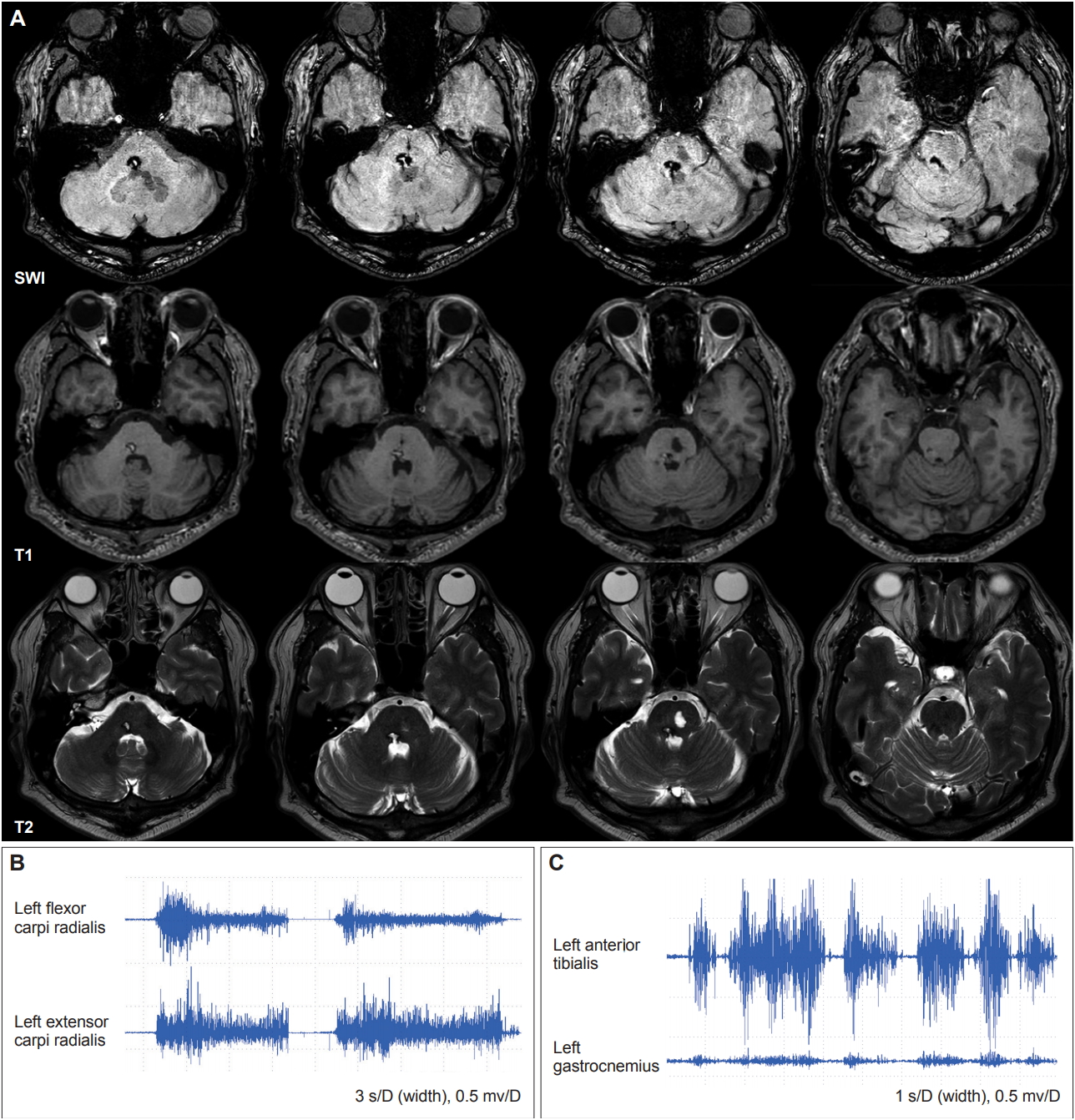
To date, there have been three reports on five cases of pontine lesions with associated hemidystonia (Table 1) [6-8]. Although the exact mechanisms underlying dystonia after pontine lesions remain uncertain, the authors proposed several explanations, including disrupted sensory afferent input to the striatum or thalamus [6], loss of pallidal inputs to the pedunculopontine fibers [6,8], and damaged dentate-rubro-olivary pathways [7,8]. In this case, some possible structures might have been involved in the pontine hemorrhagic lesion, including the medial lemniscus, central tegmental tract, and pedunculopontine nucleus.
Our patient showed severely decreased proprioception in the left extremities, which was not observed or checked in previous reports [6-8]. Abnormal proprioceptive information may not be integrated correctly into the sensorimotor system, which is linked to defective inhibition within the somatosensory cortex, causing abnormal involuntary muscle contraction [9,10]. However, it is still a challenging issue that patients with abnormal postures and a loss of proprioception can be diagnosed with true dystonia or pseudodystonia. Pseudodystonia represents abnormal postures or repetitive movements in which clinical, imaging, laboratory, or electrophysiological findings are not compatible with true dystonia [5]. The presence of associated neurological findings (e.g., sensory loss) in the affected body part is the key distinguishing feature for making a diagnosis of pseudodystonia. Moreover, in this case, eye closure accentuated the abnormal postures in the left hand, suggesting that this abnormal posture might be compensated by the visual system, unlike what occurs in true dystonia [5]. However, true dystonia may also worsen with eye closure, presumably due to distraction. Moreover, the electromyography recording showed simultaneous contraction of agonist and antagonist muscles in the left arm and leg, which is compatible with dystonia.
In conclusion, this is a rare case of hemidystonia associated with a pontine structural lesion, which might be ascribed to a loss of proprioception. Follow-up observations are needed to explore if abnormal postures can be improved with the recovery of proprioceptive sensation.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.19074.
Supplementary Video Legend
Video 1. Abnormal postures of the left hand (patterned flexion of the wrist and hyperextension of all five fingers) and left foot (patterned dorsiflexion of the ankle and flexion of the toes).
Notes
Ethical Statement
The work has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and its later amendments or comparable ethical standards for experiments involving humans. Informed consent was obtained from all the patients included in the study.
Conflict of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Seok Jong Chung and Phil Hyu Lee. Data curation: Chan Wook Park. Formal analysis: Chan Wook Park. Funding acquisition: Seok Jong Chung and Phil Hyu Lee. Investigation: Chan Wook Park. Methodology: Chan Wook Park. Supervision: Young H. Sohn. Validation: Seok Jong Chung. Visualization: Chan Wook Park. Writing—original draft: Chan Wook Park. Writing— review & editing: Seok Jong Chung and Phil Hyu Lee.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1A2A2A05920131) and the Ministry of Education (NRF-2018R1D1A1B07048959).