Neuroleptic Malignant Syndrome in a Patient with Corticobasal Degeneration
Article information
Abstract
Parkinson’s disease is a principal underlying disease of neuroleptic malignant syndrome (NMS) occurring in parkinsonian disorders, but NMS may occur in patients with progressive supranuclear palsy and multiple system atrophy. We report first patient with corticobasal degeneration (CBD) who developed NMS after abrupt reduction of antiparkinsonian medication and concurrent infection. It should be kept in mind that the prevention of infectious illness, which is common complication in parkinson-plus syndrome, is important, and dose reduction or withdrawal of anti-parkinsonian medications should be carefully performed even in the patients with CBD who are expected to be unresponsive to levodopa treatment.
Neuroleptic malignant syndrome (NMS) is characterized by high fever, worsening of parkinsonian symptoms, elevated serum creatine kinase (CK), altered consciousness, and autonomic instability.1 It is precipitated mainly by withdrawal or reduction of anti-parkinsonian medications, but infection, dehydration, poor oral intake, or even hot weather may precipitate the illness.2 Additionally, the underlying disease is not confined to Parkinson’s disease (PD), but NMS also can be found in atypical parkinsonism.2
However, there is no report of NMS occurred in patient with corticobasal degeneration (CBD). We first report a clinically diagnosed CBD patient who developed NMS after reduction of anti-parkinsonian medications and pneumonia.
Case
A 70 year old woman first visited our clinic for right hand bradykinesia and gait disturbance unresponsive to levodopa treatment. At age 67, she started to feel clumsiness in her right hand and to walk with very short stride and festination. Her gait disturbance rapidly worsened until she was unable to walk without assistance after two years. At age 69, she was diagnosed with PD and started to take levodopa.
At age 70, she took 450 mg of levodopa by regular levodopa/carbidopa, 150 mg by controlled release form of levodopa/carbidopa, 6 mg of ropinirole, and 200 mg of amantadine per day, but felt no clinical improvement. She was unable to use her right hand for daily activities. Her verbal output was markedly reduced, and she could speak only simple words. On neurological examination, she showed masked face and no rest tremor. When she stretched out her arms, her right elbow showed mild flexion dystonia. Muscle tone was moderately elevated in her right limbs and mildly in the left limbs. She could not perform hand rolling, finger tapping, and foot tapping movements with her right limbs. She could not stand still without support and fell spontaneously. Deep tendon reflexes were normoactive. The stimulus sensitive jerks and ideomotor apraxia were observed in her right hand. The unified PD rating scale motor score was 52. We were unable to check mini-mental status examination score.
A T2-weighted brain magnetic resonance imaging (MRI) study showed diffuse cortical atrophy which was prominent in the left fronto-tempo-parietal cortex, and moderate degree of hypersignal intensities in diffuse subcortical white matter. An [18F]-deoxyglucose positron emission tomography study showed severe hypometabolism in left fronto-temporo-parietal cortex, striatum and thalamus (Figure 1).
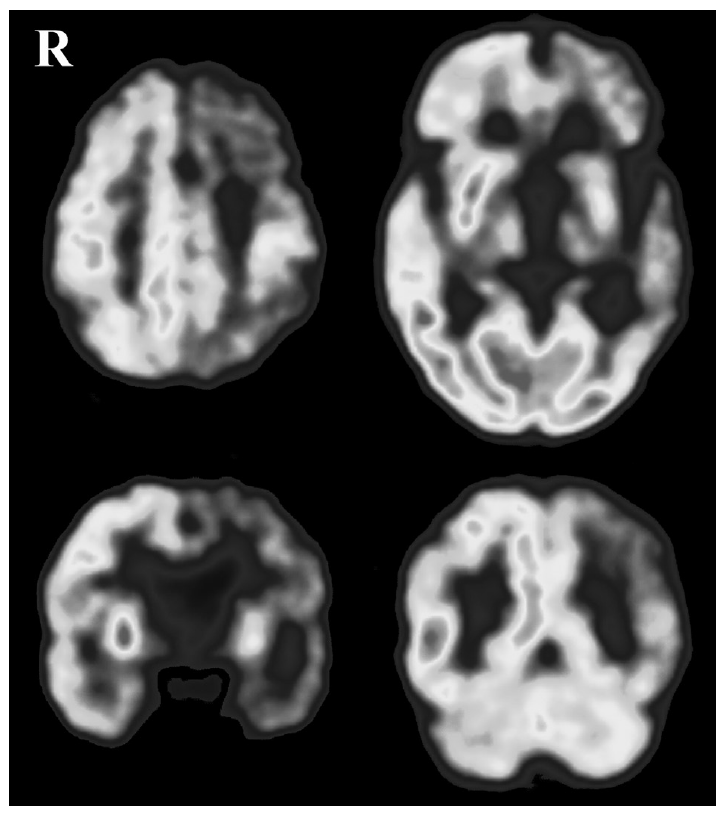
[18F]-deoxyglucose brain positron emission tomography study of the patient shows markedly reduced glucose metabolism in the left fronto-temporo-parietal cortex, left striatum and left thalamus.
Clinical diagnosis of CBD was made. Because there was no response to levodopa treatment, we started to taper off anti-parkinsonian medications. Three days after abrupt discontinuation of ropinirole, amantadine, and controlled release form of levodopa except regular levodopa/carbidopa, she developed severe dysphagia and dyspnea. Her mental status was getting drowsy and parkinsonian motor deficits rapidly exacerbated. She was bed-ridden and unable to move her body in bed. Severe rigidity was observed in her limbs and neck. Her body temperature rose up to 39ºC and heart rate over 190/min. Blood leukocyte count was 16,990/mm3. Serum CK level was 10,632 IU/L and urine myoglobin level was 2,881 ng/mL. Chest X-ray showed bronchopneumonia. There was no interval change in brain MRI study.
The dosage of levodopa was increased to 750 mg per day and antibiotics treatment was started. During the course of following seven days, body temperature and serum CK level slowly returned to the normal level. Severe rigidity of the limbs also returned to a moderate degree. However, she remained bed-ridden status even after a year.
Discussion
The PD patients with longer disease duration, higher motor severity and motor fluctuation are likely to develop NMS after abrupt reduction of anti-parkinsonian medications.3 Abrupt deterioration of dopaminergic transmission is suggested to be a common pathophysiological mechanism of NMS.2,4 However, the NMS can occur in atypical parkinsonisms, including progressive supranuclear palsy, multiple system atrophy, and CBD, which accompany the pathology in postsynaptic striatal neurons and poor levodopa responsiveness.2,5 These suggest that peripheral mechanism or the alteration of neurotransmitters other than dopamine may also be involved in the pathogenesis of NMS.2,4
In this patient, both reduction of levodopa dosage and concurrent infection might precipitate NMS. Therefore, prevention of infection, a common complication of parkinson-plus syndromes, is necessary to prevent NMS. Additionally, dose reduction or withdrawal of anti-parkinsonian medications should be carefully performed even in the patients with CBD who are expected to be unresponsive to levodopa treatment.
Acknowledgments
This work was supported by a faculty research grant of Yonsei University College of Medicine (grant number 6-2010-0016).
Notes
The authors have no financial conflicts of interest.