Hyperglycemia-Associated Hemichorea-Hemiballismus with Predominant Ipsilateral Putaminal Abnormality on Neuroimaging
Article information
Dear Editor,
Hemichorea-hemiballismus is characterized by unilateral, brief, unpredictable involuntary movements of one side of the body (upper and lower limbs and sometimes also affecting the face). The most common causes for this condition are strokes, hyperglycemia-associated hemichorea-hemiballismus, and other focal lesions affecting the basal ganglia [1]. Hyperglycemia-associated hemichorea-hemiballismus appears to be particularly common in older Asian women [2]. Brain MRI in these patients characteristically shows a hyperintense signal in the contralateral basal ganglia, particularly the putamen, on T1-weighted sequences (with variable signal intensities on T2-weighted images) [3]. T1 hyperintensity occurring exclusively or predominantly in the ipsilateral basal ganglia appears to be very rare, and to our knowledge, it has only been reported once previously in a single case [4]. We report a case of predominant ipsilateral putaminal T1 hyperintensity in a patient with diabetes presenting with hemichorea-hemiballismus secondary to nonketotic hyperglycemia.
A 76-year-old woman of Indian ancestry with diabetes mellitus and hypertension for 20 years, but no history of prior stroke, presented to a peripheral hospital in June 2018 after a fall due to acute-onset involuntary movements in the right upper and lower limb. Her blood sugar was 558.6 mg/dL (31.0 mmol/L) with no urinary ketones. She was treated initially with insulin infusion and then switched to basal-bolus insulin (total daily dose of 50 units) on discharge. Clonazepam (1.5 mg twice daily) was given for symptomatic relief. Her blood sugar levels at home were erratic with frequent hyper- and hypoglycemic episodes. The involuntary movements persisted for the next three weeks. At this time, she presented to our center with obvious right-sided hemichorea-hemiballismus with involvement of the right side of the face as well (Supplementary Video 1 in the onlineonly Data Supplement). The chorea-ballismus was aggravated by voluntary movements but absent during sleep. The left side of the body was unaffected.
During her hospital stay, her blood sugar levels fluctuated between 108.1 mg/dL (6.0 mmol/L) and 396.4 mg/dL (22.0 mmol/L). Her glycated hemoglobin (HBA1c) was 10.5%. The thyroid function test was normal. CT of the brain showed right putaminal hyperdensity. Brain MRI showed asymmetrical T1-and T2-hyperintense signals in the putamen bilaterally, which was much more prominent in the right putamen (i.e., ipsilateral to the symptomatic side), without restricted diffusion (Figure 1). A diagnosis of hyperglycemia-associated hemichorea-hemiballismus was made. She was given 1.5 mg haloperidol at night, which was gradually titrated up to 1.5 mg in the morning and 3 mg at night for symptomatic control, and her insulin treatment was adjusted. She was subsequently discharged after 14 days of hospital stay with improved glycemic control. At this time, she was able to stand with support but still had some difficulty walking.
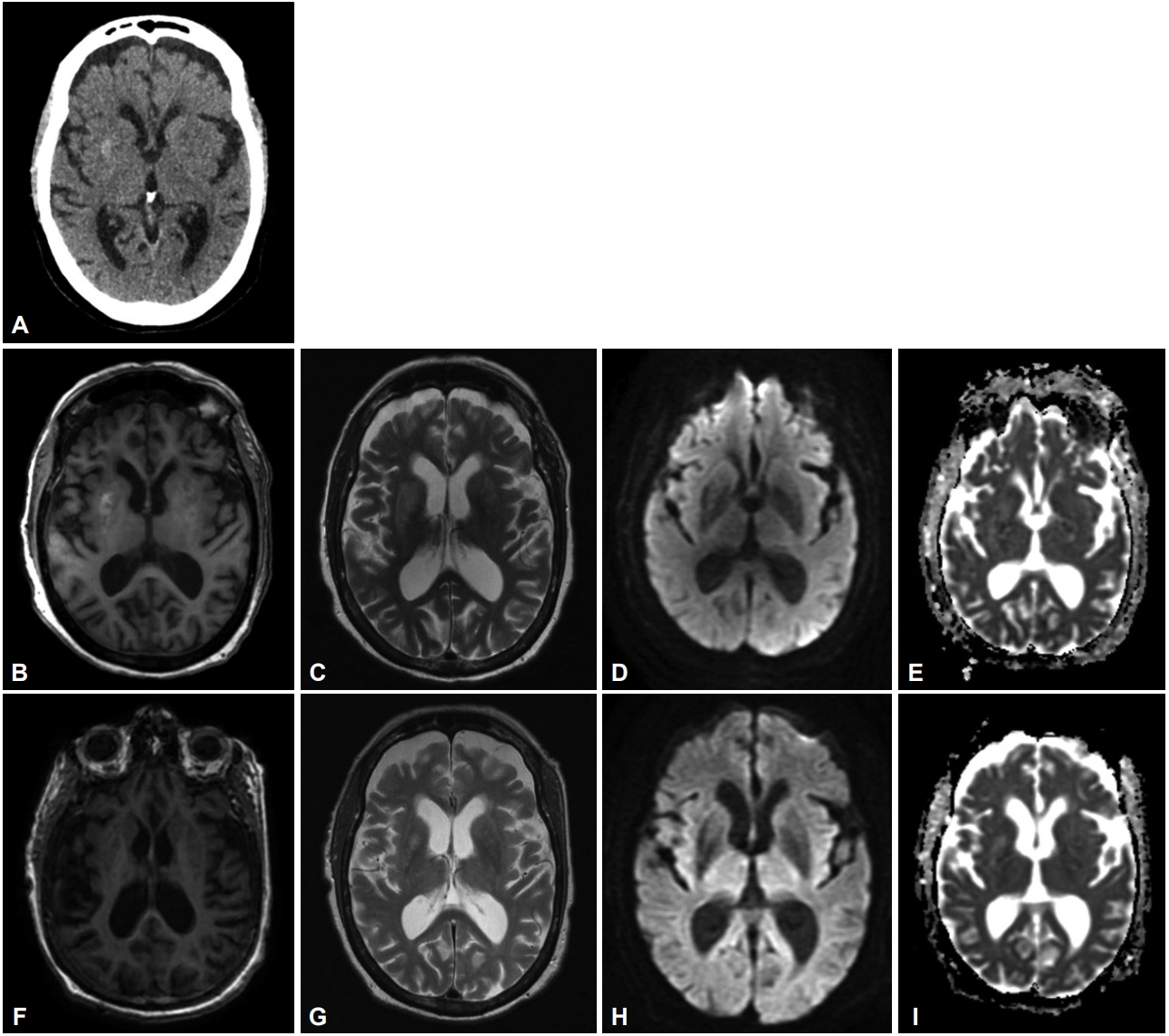
Right putaminal hyperdensity on brain CT scan (A). Bilateral putaminal hyperintensities much more marked on the right on T1- and T2-weighted sequences (B and C). No restricted diffusion on the diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps (D and E). There is no evidence of previous stroke. Follow-up images five months after initial symptom onset (F and G) show marked improvement of the signal changes with only residual hyperintensity at the right putamen on the T1 and T2 sequences. There is no restricted diffusion on the DWI and ADC maps (H and I).
Her chorea-ballismus resolved eight weeks after discharge. Therefore, her haloperidol dosage was reduced from 4.5 mg to 2.25 mg daily. She returned to the clinic for review five months after the initial symptom onset. There was no recurrence of the right-sided hemichorea-hemiballismus, and a repeat brain MRI showed marked improvement in the putaminal changes (Figure 1). However, new hyperkinetic movement disorders were observed, consisting of stereotypic forward truncal flexion movements and mild oro-buccal dyskinesias, which were diagnosed as a neuroleptic-induced tardive syndrome. A switch from haloperidol (1.5 mg morning, 0.75 mg evening) to tetrabenazine (25 mg twice daily) significantly improved her tardive syndrome.
Most patients with hyperglycemia-associated chorea-ballismus have a background of long-standing uncontrolled diabetes mellitus with HbA1c of more than 10%, as seen in this case [2]. The majority of reported patients improve within days to weeks, although female gender, extraputaminal lesions and delayed introduction of dopamine-receptor blocking agents have been associated with persistence of the movement disorder [2]. The MRI finding in our patient was unusual in that it showed predominant T1 and T2 hyperintensity in the right putamen, ipsilateral to the right-sided hemichorea-hemiballismus. The improvement of the MRI changes, in parallel with the clinical resolution of the hemichorea-hemiballismus, supports a close link between the involvement of the putamen and the clinical manifestations.
One potential explanation for this observation is that hyperglycemia, although a systemic metabolic condition, may induce a different extent of functional changes in the basal ganglia on each side, and these changes may reach the threshold for behavioral expression on only one side; however, the degree of MRI signal change may not always correlate with these functional changes. One possibility to consider is that our patient’s hemichorea-hemiballismus could be due to the contralateral basal ganglia lesion, and the more prominent right putaminal lesion was asymptomatic (particularly given that cases have been previously described that showed typical MRI lesions but did not manifest chorea-ballismus or, conversely, that presented with hyperglycemia-associated hemichorea-hemiballismus but did not show MRI lesions) [3].
Alternatively, underlying individual differences in neural pathways (e.g., nondecussation of the corticospinal tracts or shunting of excitatory input from disinhibited ipsilateral basal ganglia structures to the contralateral motor cortex via corpus callosal connections) could be relevant, as suggested by rare cases of ipsilateral hemichorea-hemiballismus occurring after stroke [5]. It is also known that the basal ganglia exert bilateral effects on motor function, as evidenced by bilateral improvement of motor features in patients with Parkinson’s disease undergoing unilateral functional neurosurgery, although clinical experience suggests that these improvements are usually much more pronounced contralateral to the side of neuromodulation [6]. Unravelling the mechanisms underlying these observations may provide further insights into basal ganglia function in both health and disease.
An additional point of interest in our patient was the development of what we believe to be a tardive stereotypy with truncal flexion movements. Typical neuroleptics, especially haloperidol, are effective and widely used to treat chorea and ballismus, albeit with potential movement disorder adverse effects such as the development of parkinsonism or tardive syndromes. Previous studies suggested that patients with diabetes may be at increased risk of developing tardive syndromes compared to nondiabetic neuroleptic-treated patients [7]; hypothesized mechanisms include micro- or macrovascular damage to the basal ganglia and impaired striatal dopamine transmission with resultant dopamine receptor hypersensitivity [7].
In conclusion, we highlight an interesting observation of a case of hyperglycemia-associated hemichorea-hemiballismus in which the clinical manifestation was ipsilateral to the predominant lesion on brain MRI. Neuroleptic treatment of the patient’s chorea-ballismus led to the development of tardive movement disorders. Further work is needed to clarify the mechanisms underlying these observations.
Supplementary Video Legends
Video 1. Segment one shows hemichorea-hemiballismus involving the right face and right upper and lower limbs. Segment two shows stereotypic forward truncal flexion movements and oro-buccal dyskinesias occurring five months after the initial onset of hemichorea-hemiballismus. The authors gratefully acknowledge the patient and her family for their consent and participation in this report, including publication of the videos.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.19014.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
