Successful Pallidal Deep Brain Stimulation in a Patient with Childhood-Onset Generalized Dystonia with ANO3 Mutation
Article information
Dystonia 24 (DYT24) is an autosomal dominant disease caused by ANO3 mutation encoding anoctamin 3 that is predominantly characterized as adult-onset craniocervical dystonia; DYT24 is frequently combined with tremor or myoclonus [1]. We recently reported a patient with the pathogenic variant of ANO3 (p.Ser651Asn) who presented with early onset generalized dystonia starting in the lower extremities at age 3 with the broadening phenotypic spectrum of DYT24 [2]. Here, we further report that the patient exhibited an excellent response to deep brain stimulation (DBS) at the internal globus pallidus (GPi).
A 17-year-old man who had been well until he was 3 years of age and then began to have intermittent left leg dystonia was referred to a movement disorder clinic for progressive gait difficulty. During the next 14 years, the generalized dystonia progressively deteriorated to involve all extremities, as well as his neck and his voice. Intermittent multifocal myoclonic jerks were noted in the neck, trunk, and extremities. At the age of 18 years, the patient became nearly wheelchair bound, but cognitive functioning was not affected, although he was behind in school with a mild attention deficit, as determined via neuropsychological testing. He underwent bilateral GPi DBS targeting the posteroventral area 15 years after symptom onset. Stimulation settings were titrated over time to a frequency of 60 Hz; a pulse width of 130 μs; an amplitude of 2.6 V; an active electrode contact of 8-9-/case+ on the right, and a frequency of 60 Hz; a pulse width of 130 μs; an amplitude of 2.5 V; and an active electrode contact of 0-1-/case+ on the left at 1 year after surgery (Figure 1A). By 12 months, he was able to read intelligibly, write in a recognizable manner, and walk with his trunk facing forward and looking ahead (Supplementary Video 1 in the online-only Data Supplement). The preoperative Burke-Fahn-Marsden Dystonia Rating Scale score was 83 (62 in movement scale, 21 in disability scale) and gradually improved to 74, 53, and 45 at 3, 6, and 12 months after operation, respectively, showing 48% improvement (Figure 1B). Quality of life was evaluated with the 36-Item Short Form Health Survey, and improvement was observed in both the physical and mental health categories (mean physical component scores of 31.9 vs. 66.8, and mean mental component scores of 66.3 vs. 78.8, preoperative vs. 12 months after operation). The Beck Depression Inventory resulted in a score of 13 preoperatively and decreased to 5 one year postoperatively. Korean form of Mini-Mental State Examination was almost unchanged (28 to 27, before and 1 year after surgery, respectively).
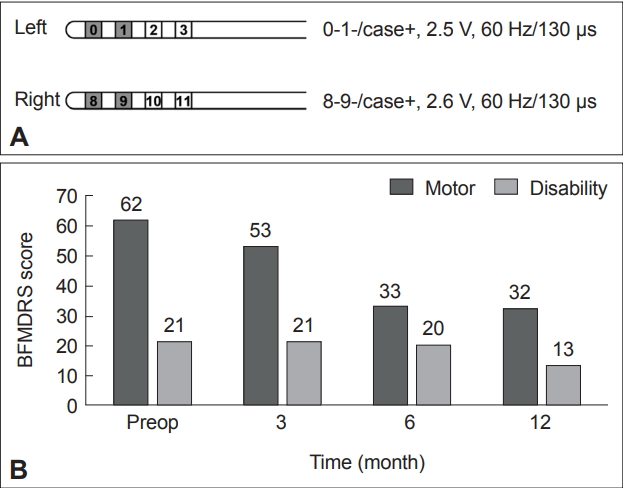
Clinical data after DBS surgery. A: Active stimulation parameters 1 year after DBS surgery. B: Time changes of BFMDRS before and 3, 6, 12 months after operation. DBS: deep brain stimulation, BFMDRS: Burke-Fahn-Marsden Dystonia Rating Scale.
The daily medication dose was reduced from 8 mg to 4 mg of trihexyphenidyl and remained at 5 mg of diazepam. The patient has not shown adverse events at the time point 1 year after surgery. This study was approved by the Institutional Review Boards of Seoul National University Hospital and written informed consent was obtained from the parents and the patient.
Zech et al. [3] briefly reported the first case of ANO3 dystonia with bilateral GPi DBS, in which a 9-year-old-onset progressive generalized dystonia exhibited a substantial improvement of dystonia and tremor after surgery at 31 year old, but details were not provided. Reporting with details, we added a case of childhood onset generalized dystonia with the pathogenic variant in ANO3, which showed a safe and effective response to bilateral GPi DBS. Although genetic factors influence DBS outcomes in dystonia [4], the consensus recommends the treatment of dystonia with DBS based on the clinical presentation [5]. In this context, patients with medically refractory isolated generalized dystonia could be referred for the consideration of pallidal neurostimulation, regardless of confirmed monogenic causes.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.19016.
Supplementary Video Legends
Video 1. Patient with ANO3 mutation before and 1 year after deep brain stimulation.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Acknowledgements
The authors thank the patient and his family for their contribution to this research.
