N30 Somatosensory Evoked Potential Is Negatively Correlated with Motor Function in Parkinson’s Disease
Article information
Abstract
Objective
The aim of this study was to investigate frontal N30 status in Parkinson’s disease (PD) and to examine the correlation between the amplitude of frontal N30 and the severity of motor deficits.
Methods
The frontal N30 was compared between 17 PD patients and 18 healthy volunteers. Correlations between the amplitude of frontal N30 and the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score of the more severely affected side was examined.
Results
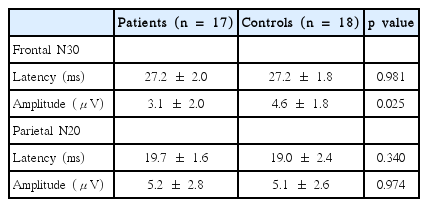
The mean latency of the N30 was not significantly different between patients and healthy volunteers (p = 0.981), but the mean amplitude was lower in PD patients (p < 0.025). There was a significant negative correlation between the amplitude of N30 and the UPDRS motor score (r = -0.715, p = 0.013).
Conclusions
The frontal N30 status indicates the motor severity of PD. It can be a useful biomarker reflecting dopaminergic deficits and an objective measurement for monitoring the clinical severity of PD.
Somatosensory evoked potentials (SEPs) are useful for evaluating the central somatosensory pathway [1,2], even in patients with normal brain MRI findings. SEP abnormalities can reflect the dysfunction of brain areas functionally connected to the sensorimotor cortex [3,4].
The frontal N30 is a negative peak in a median nerve SEP. It is associated with the motor circuit functioning (thalamo-corticobasal ganglia circuit) [5], and the regions from which the frontal N30 is generated are hypothesized to be the primary motor cortex, premotor area, prefrontal cortex, and supplementary motor area (SMA) [6,7]. The clinical significance of N30 for Parkinson’s disease (PD) has been debated, owing to contradictory results of several studies. The frontal N30 amplitude was reduced in patients with PD in some studies [8,9], but these findings were not verified in other investigations [10,11]. Initially, the frontal N30 was thought to be associated with motor function, but some previous studies failed to prove this assumption [10-12]. In two particular investigations, there was no correlation between the frontal N30 amplitude and the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score [10,12]. Additionally, there was no difference in the frontal N30 amplitude between the affected and non-affected sides [10,11].
Several factors may explain these inconsistent results, including varying study designs and populations. In particular, the number of participants may have contributed to the negative results, as most of the previous studies with negative findings were conducted with small patient populations [10,11,13]. Therefore, we investigated the difference in N30 amplitude between a comparable number of PD patients and healthy volunteers.
MATERIALS & METHODS
Participants
We recruited PD patients and healthy volunteers who visited the Department of Neurology at Hallym University Sacred Heart Hospital. PD was diagnosed according to the UK Brain Bank criteria [14]. PD patients had either asymmetric or unilateral motor symptoms. To investigate the correlation between motor deficit severity and N30 responses on the more-severely affected side, the UPDRS motor score of the more severely affected side was collected. We selected items 20–26 for the UPDRS, as these items can be measured bilaterally. The scores related to the face in item 20 and the neck in item 22 were not included, as we considered these symptoms axial. We used the UPDRS motor score for only the more severely affected side because if the frontal N30 is associated with motor function, then there should be a clear correlation between frontal N30 and the UPDRS motor score of the more severely affected side. All participants were enrolled in the study after they provided informed consent, and the study protocol was approved by the internal review of Hallym University Sacred Heart Hospital.
SEP recordings
Median nerve SEPs were recorded from all participants with an EMG machine (Neuropack 8, Nihon-Kohden, Tokyo, Japan). During the recording, participants lay in a quiet, semi-darkened room. Five of the 17 patients were de novo PD (Table 1), and the others were taking their usual medication. Each median nerve was stimulated at the wrist. The stimuli were square-wave electrical pulses with a 0.2-ms duration and applied at a frequency of 2 Hz. The stimulus intensity was determined so that it produced a painless contraction of the abductor pollicis brevis muscle. The amplifier bandpass was 20–3000 Hz.
SEPs were recorded with Ag/AgCl disc electrodes placed on the scalp at F3/F4, C3/C4 according to the international 10–20 system. Each electrode was referenced to linked earlobes. At least 500 stimuli were applied for each recording. Recordings were performed at least twice to confirm behavior. N20 and P25 waves were recorded from the C3/C4 electrodes, whereas the P22 and N30 waves were recorded from the F3/F4 electrodes. The peak latency was measured from the onset of the stimulus artifact. The peak-to-peak amplitude was also measured.
Statistical analysis
Data were expressed as the mean ± standard deviation. The mean age, mean peak latency values, and peak-to-peak amplitudes were compared between PD patients and healthy volunteers with unpaired t-tests. Sex was compared between patients and healthy volunteers with Fisher’s exact test. Spearman’s correlation was used to evaluate the response to stimulation for the more severely affected side and the UPDRS motor score. Values of p < 0.05 were considered significant.
RESULTS
Seventeen PD patients (mean age: 65.7 ± 8.8 years; 9 women) and 18 healthy volunteers (mean age: 61.1 ± 12.0 years; 11 women) participated in this study. There was no significant difference in age (p = 0.217) or sex (p = 0.738) between the two groups. The clinical features of the PD patients are summarized in Table 1. The UPDRS motor score was obtained from 11 patients.
The mean latency and amplitude values for the frontal N30 and parietal N20 are shown in Table 2. We obtained the N20 in all patients and controls, but were unable to obtain the N30 on the more-severely affected side in three PD patients. There was no significant difference in the mean latency of N30 between patients and controls (p = 0.981), but the mean amplitude of N30 was lower in PD patients (p = 0.025). No significant differences between patients and controls were observed in the N20 latency and amplitude (Figure 1).
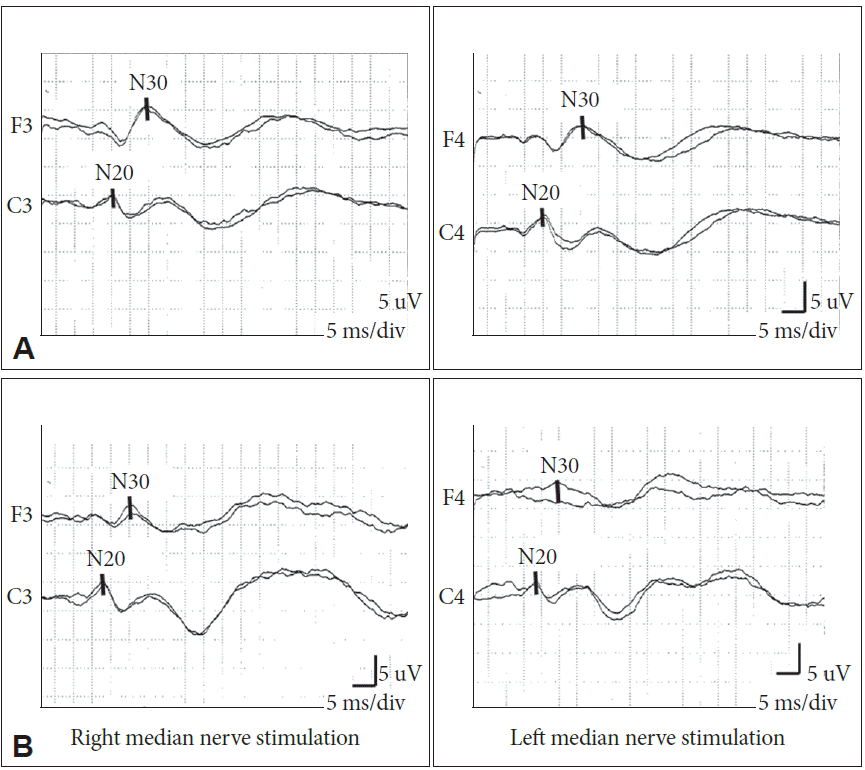
Frontal N30 and parietal N20 SEPs evoked with median nerve stimulations in (A) a healthy participant and (B) a PD patient. SEPs: somatosensory evoked potentials, PD: Parkinson’s disease.
A correlation analysis was performed in 11 PD patients. The amplitudes of N30 and UPDRS motor scores were significantly and negatively correlated on the more severely affected side (r = -0.715, p = 0.013) (Figure 2).
DISCUSSION
Our study demonstrates that the frontal N30 represents the motor dysfunction of PD patients. The amplitudes were lower in PD patients than in healthy volunteers and were closely correlated with motor deficit severity on the more-severely affected side of PD patients.
The frontal N30 amplitude reflects the functional connectivity of sensorimotor integration, which includes the thalamus, premotor area, basal ganglia and primary motor cortex [15]. The precise location of this activity is still unknown, but it is thought to originate in the SMA [16,17]. Frontal N30 activity was attenuated in meningioma compressing the SMA [18], and intracortical recordings showed that N30 activity was most evident within the premotor cortex [16]. The increase in N30 amplitude seems to result from increased neuronal activity in these regions, as the N30 amplitude increases following repetitive motor tasks [15]. The increase in N30 amplitude may be a result of cortical inhibition rather than facilitation [19].
Our results (low N30 amplitude in PD) might be due to decreased activity in the SMA. In PD, the activity in the SMA is reduced, but this can be reversed with antiparkinsonian medication (i.e., apomorphine, levodopa) [20,21]. The negative correlation we found between the N30 amplitude and motor deficit severity suggests that the N30 might be associated with dopaminergic activity. In previous studies, the N30 amplitude increased with dopaminergic medications, which also supports our assumption [22,23]. Two such studies indicated the N30 amplitude increased after apomorphine [22] and was positively correlated with plasma levels of levodopa [23].
As described previously, explanations for the negative results in prior studies of the relationship between PD and motor deficit severity remains unclear. One potential explanation for the negative results is the use of small sample sizes. We addressed this issue in our present study by using larger experimental and control populations than those used in previous investigations. There may be additional explanations for these negative results. Generally, the patient population might be a contributing factor (i.e., age, disease duration, etc.). We summarized the characteristics of the patient populations in previous studies (Table 3). We also speculate that other factors might modulate the amplitude of the frontal N30. The frontal N30 is reduced during motor preparation and planning, observation, and imaginary movement, as well as with sensory inputs including touch and proprioception [24-27]. In fact, because we did not give specific instructions about these factors (i.e., observation, thought) to our patients during SEP recording, we do not know whether these are indeed potential confounders, which remains to be investigated.
The frontal N30 amplitude presents sensorimotor integration, possibly relating to dopaminergic function. Our study indicates that the frontal N30 amplitude is lower in PD and is negatively correlated with motor deficit severity. The frontal N30 can be a useful ancillary biomarker for monitoring the clinical severity of PD. Additional work is needed to further examine the characteristics of the frontal N30 and its contributing factors.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.