The Frequency and Severity of Gastrointestinal Symptoms in Patients with Early Parkinson’s Disease
Article information
Abstract
Objective
Although gastrointestinal dysfunctions occur in the majority of patients with Parkinson’s disease (PD), they are often unrecognized because many patients remain relatively asymptomatic in the early stage. We investigated the frequency of gastrointestinal symptoms in patients with PD using newly developed gastrointestinal symptom questionnaires.
Methods
Early PD patients with a symptom duration not exceeding 3 years were included in this study. All PD patients were evaluated using a questionnaire, which consisted of three relevant domains: oropharyngoesophageal (10 items); gastric (3 items); and intestinal-anorectal (7 items). The frequency of symptoms was calculated as a proportion with an item score ≥ 2.
Results
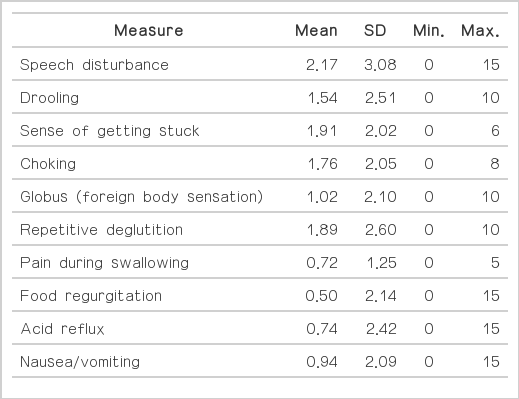
Of the 54 patients enrolled, 48 patients (88.9%) responded that bowel symptoms developed before the onset of Parkinsonian motor symptoms, and four patients reported that the onset of two types of symptoms (i.e., bowel and neurological) occurred approximately simultaneously, with only months between them. The frequencies of gastrointestinal symptoms are as follows: speech disturbance (40.7%), drooling (24.1%), sense of getting stuck (31.5%), choking (27.8%), globus pharyngis (16.7%), repetitive deglutition (29.6%), pain during swallowing (5.6%), food regurgitation (3.7%), acid reflux (7.4%), nausea/vomiting (11.1%), early satiety (16.7%), postprandial fullness (14.8%), epigastric soreness (9.3%), abdominal pain (3.7%), constipation (46.3%), excessive strain during defecation (33.3%), fecal incontinence (7.4%), tenesmus (20.4%), loose stool or diarrhea (3.7%), and difficulty in relaxing anal sphincter (11.1%). Two patients were scored at zero.
Conclusions
Our findings confirm that gastrointestinal dysfunction occurs in early PD in relatively high frequency.
Gastrointestinal (GI) symptoms are relatively common in patients suffering from Parkinson’s disease (PD), and these symptoms can be a significant burden on patients and their caregivers. Recent advances in neuropathology address the role of autonomic nuclei in the brainstem and the enteric nervous system in the control of gastrointestinal function, explaining early involvement of this function in PD.1,2
The under-recognition and low levels of awareness of the presence of gastrointestinal dysfunctions by patients, caregiver, and health professionals result in underreporting and a low frequency of complaints. Early detection and effective intervention can help prevent the serious consequences in the quality of life. Several questionnaires have previously been developed for screening GI symptoms;3–6 however, many of these questionnaires relied on specific, subjective complaints and did not include many types of symptoms.
We developed new gastrointestinal symptom questionnaires and evaluated the prevalence of gastrointestinal symptoms in early PD patients.
METHODS
Patients
Consecutive early PD patients were enrolled from the Movement Disorders Clinic at Seoul St. Mary’s Hospital, Seoul, Korea. A clinical diagnosis of PD was made according to the UK brain bank criteria.7 None of the patients had ever taken medication for PD. The evaluation procedure included a detailed medical and drug history, a physical and neurological examination and a brief neuropsychological assessment. Excluded from the study were patients with the following: 1) neurological abnormalities related to atypical PD or secondary Parkinsonism, 2) patients with diabetic neuropathy, and 3) patients taking medications known to influence gastrointestinal motility, such as levodopa, dopamine agonists, anticholinergics, or GI dysmotility remedies.
This study was approved by the local ethics committee, and all subjects gave their consent to participate.
Clinical assessments
All patients were subsequently evaluated using the Unified Parkinson’s Disease Rating Scale (UPDRS), the Hoehn and Yahr (H&Y) stage, and the Korean version of the Schwab and England activities of daily living. The patients were also asked to complete a self-administered scale to evaluate the presence and frequency or severity of bowel symptoms during the last 3 months. The self-administered questionnaire consisted of the following 20 items (Table 1): 1) speech disturbance, 2) drooling, 3) sense of getting stuck, 4) choking, 5) globus pharyngis, 6) repetitive deglutition, 7) pain during swallowing, 8) food regurgitation, 9) acid reflux, 10) nausea/vomiting, 11) early satiety, 12) postprandial fullness, 13) epigastric soreness, 14) abdominal pain, 15) constipation, 16) excessive strain during defecation, 17) fecal incontinence, 18) tenesmus, 19) loose stool or diarrhea, and 20) difficulty in relaxing anal sphincter. Each symptom was scored with respect to either the frequency [1 = rare (< 1/week), 2 = often (1/week), 3 = frequent (several times per week), and 4 = always (daily or all the time)] or severity (0 = no or minimum, 1 = mild symptoms present but causing little distress or disturbance, 2 = moderate symptoms causing some distress or disturbance, and 3 = severe symptoms representing a major source of distress or disturbance) of the symptom. The overall score was calculated by multiple severity and frequency scores.
Data analysis
Patients were categorized into the following two subgroups: 1) patients with no or mild problems (a score for each item of ≤ 1) and 2) patients with moderate-to-severe problems (a score for each item of ≥ 2), and the frequencies of each symptom were calculated using this categorical variable. Items were also grouped into three relevant domains: oropharyngoesophageal (10 items), gastric (3 items), and intestinal-anorectal (7 items). The symptom scores were also compared with Parkinsonian motor symptoms. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA), and the statistical significance level was established at p < 0.05.
RESULTS
Among the 54 patients enrolled, 22 were men and 32 were women. The mean age at examination was 67.1 ± 10.3 years (range, 33–86 years) and the mean motor symptom duration was 14.7 ± 9.3 months (range, 1–36 months). The severity of Parkinsonian motor symptoms was as follows: mean total UPDRS score was 25.1 ± 18.6 (part 1, 2.3 ± 2.3; part 2, 8.3 ± 6.9; part 3, 14.4 ± 10.6), and the mean H&Y stage was 1.6 ± 0.4. Fourteen patients were scored as H&Y stage 1, 20 patients as stage 1.5, and 20 patients as stage 2.
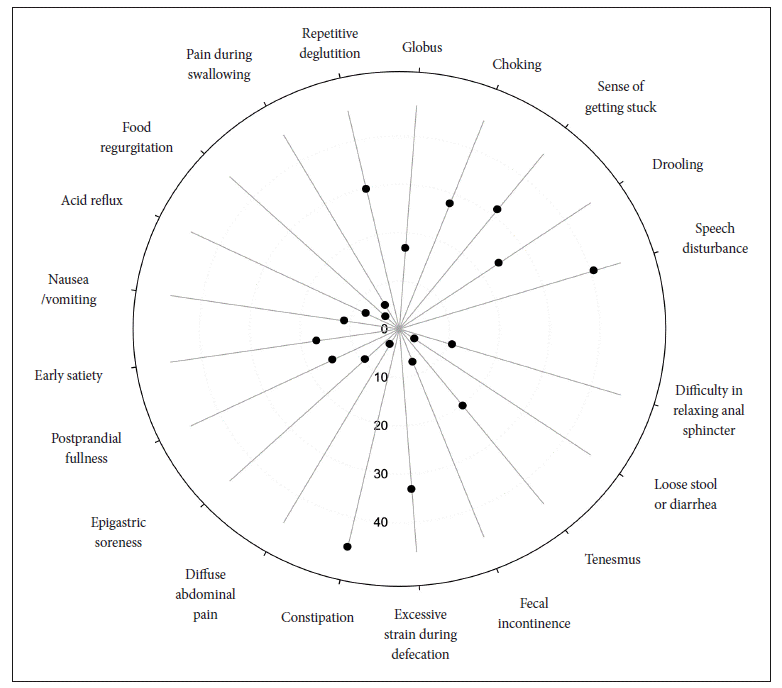
The frequencies of gastrointestinal symptoms are as follows (Table 1, Figure 1): speech disturbance (40.7%), drooling (24.1%), sense of getting stuck (31.5%), choking (27.8%), globus (16.7%), fragmented deglutition (29.6%), pain during swallowing (5.6%), food regurgitation (3.7%), acid reflux (7.4%), nausea/vomiting (11.1%), early satiety (16.7%), postprandial fullness (14.8%), epigastric soreness (9.3%), abdominal pain (3.7%), constipation (46.3%), excessive strain during defecation (33.3%), fecal incontinence (7.4%), tenesmus (20.4%), loose stool or diarrhea (3.7%), and difficulty in relaxing anal sphincter (11.1%). Only two patients scored 0 on the self-administered scale. Forty-eight patients (88.9%) responded that the bowel symptoms developed before the onset of Parkinsonian motor symptoms and worsened after the onset, and four patients reported that the onset of two types of symptoms (i.e., bowel and neurological) occurred approximately simultaneously, with only months between them.
The gastrointestinal symptoms were positively correlated with the UPDRS scores and, in particular, the activity of daily living (ADL) portion of the scores (Figure 2). However, neither the specific motor subtype nor the disease duration was associated with the severity of bowel symptoms.
DISCUSSION
The results of this study suggest that bowel symptoms are present in a majority of early, non-treated PD patients, even before the onset of clinical motor symptoms. The most frequent symptoms found in our patients were difficulty with deglutition and constipation. Dysphagia in patients with PD may be associated with abnormalities found during the oral to esophageal stages of swallowing.8 Dysphagia can result from the motor symptoms of PD involving the oropharyngeal muscles or can occur from defective coordination of the oropharyngeal and esophageal musculature resulting from brainstem involvement.9 About half of the patients with early PD in our study reported a variety of complaints associated with dysphagia. In particular, repetitive deglutition was predominantly related to oropharyngoesophageal dysfunction. Recently, we clearly showed that a majority of early PD patients underwent repetitive swallow attempts (double or triple swallow pattern) for a single bolus.8 These abnormal swallow patterns were shown to be directly associated with abnormal peristalsis in the esophageal manometry. Repetitive deglutition might be a reactive response necessary to clear remnant bolus material on the pharynx. Such a repetitive deglutition was closely related to esophageal dysfunction though the vagal inhibitory dysfunction. Under normal physiological conditions, while there are repetitive swallow attempts, esophageal peristalsis only responds to the last stimuli, called deglutitive inhibition. Normal deglutitive inhibition indicates intact vagal inhibitory function. Deglutition-evoked peristaltic sequences are mediated via vagal efferent nerves, and peristalsis occurs with active inhibition followed by sequential esophageal contractions that are centrally mediated.10 Therefore, repetitive deglutition may be the most relevant dysfunction, due to its association with esophageal dysmotility, and the above described abnormal deglutitive inhibition provides further evidence of neuronal degeneration of the brain stem swallowing center or abnormalities of the peripheral vagus nerve in patients with early PD.8
Early-stage PD patients in our study showed dysfunctions of the proximal part of the gastrointestinal system, encompassing the stomach and small intestine. Prior reports have suggested that impaired gastric motility is associated with different medications used for the treatment of PD, especially in those patients with response fluctuation. Although major pathological changes in the enteric nervous system were described, some previous studies indicate that the presence of gastroparesis in only 7.5% of PD patients was related to PD itself.11,12 In this study, we show that the proportions of gastric symptoms ranged from 3.7% to 16.7%. Gastric symptoms are a well-recognized manifestation of PD, which can cause not only nausea and other symptoms, such as gastroparesis, but can also affect drug absorption.13 In addition, antiparkinson medications may further exacerbate these symptoms. Therefore, it is important that clinicians help patients with PD find and overcome these disabling symptoms.
The most common gastrointestinal symptoms in our study were related to constipation and difficulty in defecation. Constipation and defecatory dysfunction in patients with PD may be associated with abnormalities occurring during colonic inertia to the anal outlet.14 Defecatory dysfunction is a consequence of uncoordinated action of the muscles involved with defecation. Relaxation of the puborectalis muscle allows for the opening of the anorectal angle and perineal descent, facilitating fecal expulsion. This functional outlet obstruction may cause both excessive straining and a sense of incomplete emptying, or sometimes, may induce painful defecation. 15–17 Another mechanism appears to involve the colonic musculature, which induces a slow passage of feces through the colon.14 Several studies have demonstrated considerably prolonged mean colonic transit time in PD patients.18
Gastrointestinal symptoms were correlated with the UPDRS score, particularly the ADL score. This suggests that the severity of motor symptoms might be closely related to enteric involvement, which is distinct from the degree of nigrostriatal involvement, and other neurotransmitter systems can be selectively affected.
In summary, most of the included patients with early PD had several instances of gastrointestinal symptoms. Many patients reported that these symptoms developed before the clinical motor manifestation of PD. These findings suggest selective involvement of either the brain stem and/or the enteric nervous system at an early stage of PD. The more severe and frequent gastrointestinal symptoms were associated with severe motor symptoms but did not correlate with the duration of PD. Comprehensive screening of gastrointestinal symptoms in patients with early-stage PD can facilitate prompt recognition and effective therapeutic interventions for significant bowel dysfunctions.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.