ABSTRACT
- A decreased cardiac 123I-metaiodobenzylguanidine (123I-MIBG) uptake has been used as a powerful tool to identify Lewy body disease, such as idiopathic parkinson’s disease (IPD). We performed cardiac 123I-MIBG scintigraphy in patient with autosomal recessive juvenile parkinsonism (ARJP) with parkin gene mutation (PARK2). The findings showed normal cardiac 123I-MIBG uptake. Therefore, although the clinical features of ARJP are sometimes quite similar to those of late-onset IPD, cardiac 123I-MIBG scintigraphy may be used as a valuable tool to identify patients with IPD and to distinguish them from patients with other parkinsonian syndromes.
-
Keywords: 123I-metaiodobenzylguanidine; Autosomal recessive juvenile parkinsonism; Parkin gene
Cardiac 123I-metaiodobenzylguanidine (MIBG) scintigraphy has been investigated as a useful tool to distinguish Parkinson’s disease (PD) from atypical Parkinsonism.1 This feature corresponds to the presence of myocardial postganglionic sympathetic dysfunction as part of the neurodegenerative process in PD. This impairment occurs early in the course of the disease, and its severity depends on disease progression and treatment. Parkin disease (PARK2; OMIM 602544) is the most frequent monogenic early-onset parkinsonism (EOP), accounting for approximately 49% of familial EOP.1 Pathological features of parkin disease resemble those of idiopathic Parkinson’s disease (IPD) in that they show degeneration of neurons in the substantia nigra pars compacta and in the locus coeruleus2, and occasionally Lewy bodies. 2,3 The typical clinical phenotype shows early onset of parkinsonian features, usually before the age of 40, benign clinical course, dystonia at onset, increased tendon jerks, sleep benefit, early motor fluctuations, and susceptibility to levodopa-induced dyskinesias. Recently, it has been suggested that cardiac 123I-MIBG scintigraphy could be used as a tool to estimate Lewy body pathology and to distinguish IPD from this disorder. Study about cardiac 123I-MIBG uptake in a patient with parkin disease was not established yet in the Korean population. Thus, we herein report the cardiac 123I-MIBG scintigraphy findings of a patient with Korean familial parkin disease.
Case
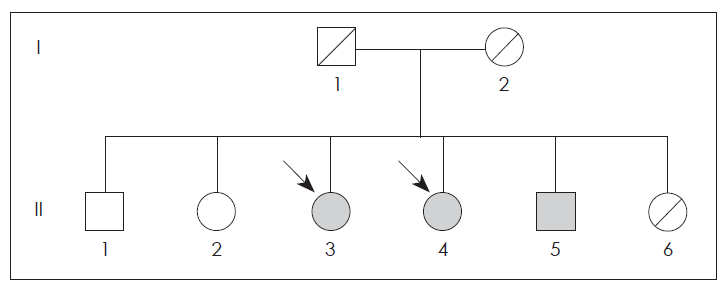
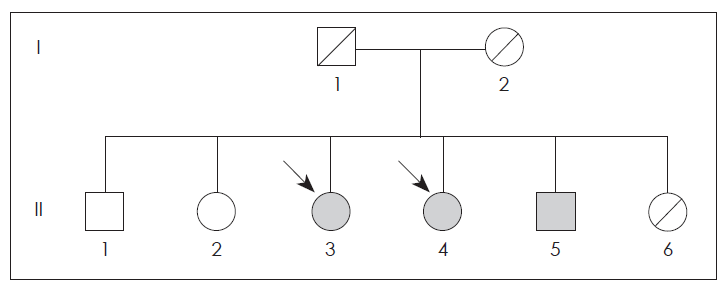
- This family was briefly described as having familial parkin disease by Kim et al.4,5 (Figure 1). The present patient (II-4, born 1954) noticed a bilateral resting tremor at 38 years of age. When bradykinesia and gait disturbance developed later, she was diagnosed with PD. The disease progressed slowly, affecting both sides of the body.
- She was treated with 750 mg levodopa/carbidopa, which resulted in marked improvement in her parkinsonian features. The Hoehn and Yahr stage on state was I. She additionally showed only mild dysarthria on neurological examination at 47 years of age, but the brain magnetic resonance imaging was normal finding. However, right foot and trunk dyskinesia (peak dose dyskinesia) was marked at 50-years of age, and we reduced dose of levodopa/carbidopa to 500 mg.
- The older sister (II-3, born 1950) noticed a gait disturbance at age 44 and was diagnosed with Parkinson disease. The younger brother (II-5, born 1958) presented with paresthesia of the lower extremities and twisting sense in the left toe at the age of 34, but he did not exhibit any parkinsonism during 10-years follow up period. Another brother (II-1, born 1939) was normal. The PCR amplification of the family members revealed a homozygous deletion of exon 4 in the parkin gene (II-3, II-4 and II-5) (Figure 1).
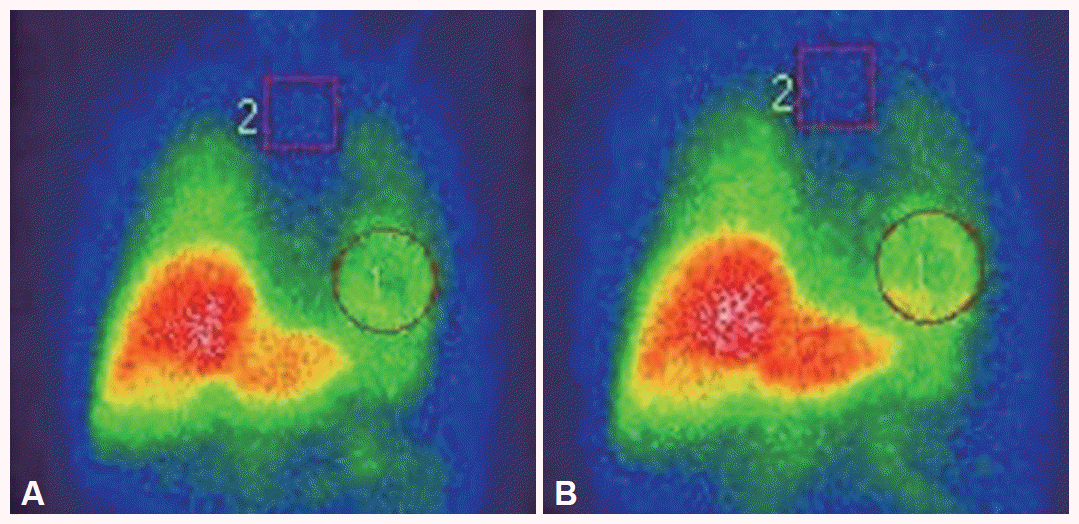
- The cardiac 123I-MIBG scintigraphy of the patient was performed with an intravenous injection of 111 MBq of 123I-MIBG using a dual-head camera (Siemens, Germany). Data of early and delayed phases was obtained 30 minutes (early) and 4 hours (delayed) after injection. The values of the heart to mediastinum (H/M) ratio in the early and delayed phases were 1.94 and 1.99, respectively (Figure 2). These ratios were significantly higher than the 2SDs calculated from 50 PD patients (early; 1.343 ± 0.251, delayed 1.258 ± 0.242) and were was within 2 SDs of the normal mean (early; 2.342 ± 0.214, delayed; 2.418 ± 0.231).
Discussion
- A reduced cardiac uptake of MIBG, which indicates cardiac sympathetic denervation, may occur even during the early stage and offers a sensitive tool with which to differentiate PD and diffuse Lewy body dementia (DLB) from other disorders with akinetic rigid symptoms and dementia.3 All of IPD, DLB and pure autonomic failure have Lewy bodies as a common pathologic feature and are considered to be 3 phenotype of single disorder that may be called Lewy body disease (LBD).2 Whereas, neuropathological studies have revealed the generalized loss of neurons with no Lewy bodies in the substantia nigra and locus ceruleus of most case of parkin disease. Our patient showed normal cardiac uptake of 123I-MIBG. The present findings and previous reports, even considering the limited number of patients, strongly suggest that cardiac uptake of MIBG is a useful diagnostic marker to differentiate parkin disease from PD.
- The present data raise the issue whether normal cardiac sympathetic innervation is a feature of parkin disease. In previous study, Mashiko et al.6 reported that myocardial MIBG scan was normal in Japanese autosomal recessive juvenile parkinsonism patient caused by parkin gene. Although involvement of cardiac sympathetic nerve system in parkin disease is unknown, to our knowledge, this is the first report of cardiac 123I-MIBG scintigraphy finding in Korean familial parkin disease. Therefore, we think normal cardiac uptake of 123I-MIBG might be of potential diagnostic value to indicate the absence of Lewy body pathology, resulting in distinguishing LBD, such as IPD and DLB, from other diseases without Lewy body pathology, including parkin disease.
Notes
-
The authors have no financial conflicts of interest.
Figure 1Cardiac 123I-metaiodobenzylguanidine scintigraphy in this patient. All of early (A) and delayed images (B) reveal normal cardiac 123I-metaiodobenzylguanidine uptake. The H/M ratio is 1.94 in the early image and 1.99 in the delayed image. H/M: heart to mediastinum.
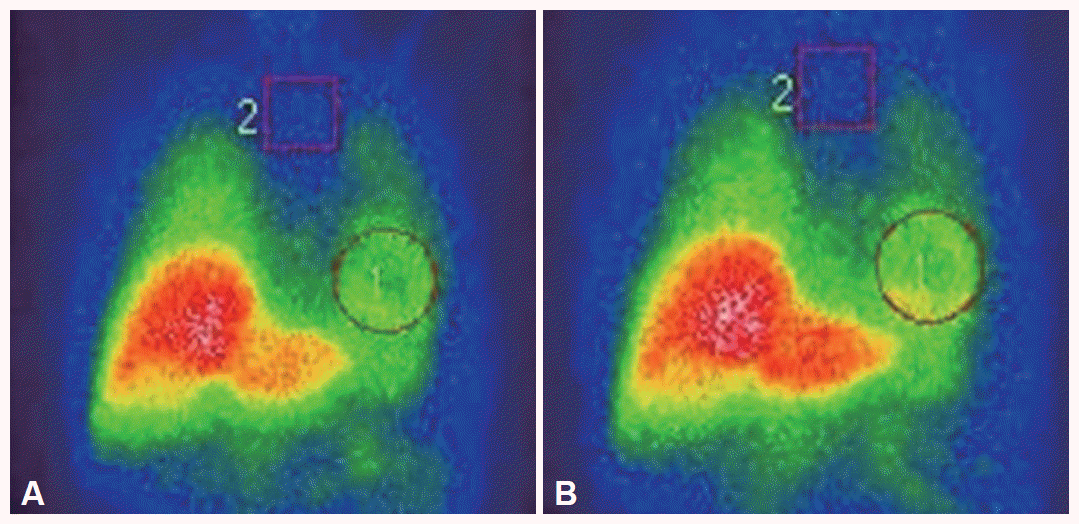
Figure 2Pedigree of family. Circle indicate women, squares indicate men and arrows indicate the affected individuals. Black symbols represent persons with homozygous mutations. Hatched symbols denote deceased subjects.
REFERENCES
- 1. Kashihara K, Yamamoto M. Myocardial 123I- MIBG scintigraphy in patients with PSP, CBD and MSA. J Neurol 2006;253(Suppl 3):III/35–III/40.Article
- 2. Kashihara K, Ohno M, Kawada S, Okumura Y. Reduced cardiac up-take and enhanced washout of 123I-MIBG in pure autonomic failure occurs conjointly with Parkinson’s disease and dementia with Lewy Bodies. J Nucl Med 2006;47:1099–1101.PubMed
- 3. McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872.ArticlePubMed
- 4. Kim JS, Lee KS, Kim YI, Lee KH, Kim HT. Homozygous exon 4 deletion in parkin gene in a Korean family with Autosomal recessive early onset parkinsonism. Yonsei Med J 2003;44:336–339.ArticlePubMed
- 5. Kim JS, Kim KS, Park SK, Lee KS. Loss of sriatal dopamine transporter binding in patent and a family member with familial parkinsonism with parkin gene mutation. J Korean Neurol Assoc 2005;23:132–134.Article
- 6. Suzuki Masahiko, Hattori Nobutaka, Orimo Satoshi, Fukumitsu Nobuyoshi, Abo Masahiro, Kono Yu, et al. Preserved Myocardial [123I] metaiodobenzylguanidine uptake in autosomal recessive juvenile parkinsonism: first case report. Mov Disord 2005;20:634–636.ArticlePubMed
Citations
Citations to this article as recorded by

- Parkinsonism in spinocerebellar ataxia with axonal neuropathy caused by adult-onset COA7 variants: a case report
Shogo Ouchi, Kazuhiro Ishii, Kenjiro Kosaki, Hisato Suzuki, Mamiko Yamada, Toshiki Takenouchi, Akira Tamaoka
BMC Neurology.2023;[Epub] CrossRef - α‐Synuclein Deposition in Sympathetic Nerve Fibers in Genetic Forms of Parkinson's Disease
Risa Isonaka, David S. Goldstein, William Zhu, Esther Yoon, Debra Ehrlich, Alice B. Schindler, Angela D. Kokkinis, Marya S. Sabir, Sonja W. Scholz, Sara Bandres‐Ciga, Cornelis Blauwendraat, Pedro Gonzalez‐Alegre, Grisel Lopez, Ellen Sidransky, Derek P. Na
Movement Disorders.2021; 36(10): 2346. CrossRef - Cardiac sympathetic burden reflects Parkinson disease burden, regardless of high or low orthostatic blood pressure changes
Sang-Won Yoo, Joong-Seok Kim, Yoon-Sang Oh, Dong-Woo Ryu, Seunggyun Ha, Ji-Yeon Yoo, Kwang-Soo Lee
npj Parkinson's Disease.2021;[Epub] CrossRef - Normal ‘heart’ in Parkinson's disease: is this a distinct clinical phenotype?
J.‐S. Kim, H.‐E. Park, I.‐S. Park, Y.‐S. Oh, D.‐W. Ryu, I.‐U. Song, Y.‐A. Jung, I. R. Yoo, H.‐S. Choi, P. H. Lee, K.‐S. Lee
European Journal of Neurology.2017; 24(2): 349. CrossRef


 KMDS
KMDS
 E-submission
E-submission


 PubReader
PubReader ePub Link
ePub Link Cite
Cite