Articles
- Page Path
- HOME > J Mov Disord > Volume 16(1); 2023 > Article
-
Viewpoint
Mask on, Mask off: Subclinical Parkinson’s Disease Unveiled by COVID-19 -
Milan Beckers

 , Bastiaan R Bloem
, Bastiaan R Bloem , Rick C Helmich
, Rick C Helmich
-
Journal of Movement Disorders 2023;16(1):55-58.
DOI: https://doi.org/10.14802/jmd.22067
Published online: November 11, 2022
Center of Expertise for Parkinson & Movement Disorders, Department of Neurology, Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands
- Corresponding author: Milan Beckers, MD, MSc Center of Expertise for Parkinson & Movement Disorders, Department of Neurology, Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen Medical Center, PO Box 9101, 6500 HB Nijmegen, The Netherlands / Tel: +31-24-3613392 / Fax: +31-24-3618837 / E-mail: Milan.Beckers@Radboudumc.nl
Copyright © 2023 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Since early in the coronavirus disease (COVID-19) pandemic, the neuroinvasive potential of the SARS-CoV-2 virus has been recognized. Common neurological complaints experienced during COVID-19 are headache, dizziness, hyposmia and hypogeusia. The latter symptoms are thought to originate from viral invasion of the olfactory bulb via angiotensin-converting enzyme 2 (ACE2) receptors [1]. It has been proposed that SARS-CoV-2 has the potential to cause Parkinson’s disease (PD), analogous to the epidemic of encephalitis lethargica and postencephalitic parkinsonism that followed the Spanish flu pandemic in the early 20th century [2]. Excluding cases of transient parkinsonism in the context of encephalopathy, five cases of PD diagnosed in a short temporal relationship with a COVID-19 episode have been reported in the literature thus far [3-6]. Furthermore, two cases have been reported describing encephalopathy with more extensive neurological features than just parkinsonism, which were accompanied by asymmetrical abnormalities on dopamine transporter single-photon emission computed tomography (DaT-SPECT) [7,8]. Para- or postinfectious parkinsonism is plausible from both a pathophysiological and a clinical standpoint: in one of the cases, a (partial) spontaneous resolution was described, which would appear to be more consistent with an inflammatory rather than a degenerative origin [7]. Interestingly, one of the described cases [8] followed a biphasic course, comprising an acute phase of encephalopathy with myoclonus, followed by the development of an asymmetric hypokinetic-rigid syndrome one month after discharge, with DaT-SPECT demonstrating an asymmetric presynaptic dopaminergic deficit in the striatum. This course could be regarded as suggestive of postencephalitic parkinsonism.
- In the other cases of post–COVID-19 PD described thus far, however, there have been insufficient grounds to rule out the “unmasking” of already present subclinical PD rather than de novo PD caused by SARS-CoV-2 [9]. One of the patients had preexisting constipation, which might be regarded as a nonmotor prodromal symptom [5], and two patients had genetic mutations predisposing them to PD [6]. Here, we report two new cases of de novo PD after SARS-CoV-2 infection. In both cases, the presence of subtle prodromal symptoms suggests that latent PD was unmasked by COVID-19. We also discuss how a concurrent SARS-CoV-2 infection might lead to this unveiling of underlying PD.
INTRODUCTION
- Both patients were right-handed, 50-year-old women with clear past medical histories and no previous medication use. They had COVID-19 in March 2020. Both patients exhibited mild (respiratory) symptoms and were not admitted to the hospital. Their diagnoses were confirmed by positive antibody tests; polymerase chain reaction tests were not available to nonhospitalized patients at the time. Neither of the two patients had symptoms suggestive of nervous system involvement (e.g., hyposmia or hypogeusia), apart from headache.
- After the COVID-19 episode, Patient 1 noticed ‘pins and needles’ and loss of control over her right hand, with writing difficulties that gradually evolved into micrographia. In retrospect, these complaints had already been present to a lesser degree two years prior to her COVID-19 episode, but the speed of progression increased after the COVID-19 episode. She also developed a restless and numb feeling of her right leg without gait difficulties. Patient 2 noticed fatigue and a slowness of movement of her left arm and leg. She could not remember the interval between the COVID-19 symptoms and the onset of neurological symptoms. There had been no obvious progression of symptoms over the past year. The patient reported that since a year prior to the COVID-19 episode, she had been walking ‘funnily,’ and her left arm swing had been reduced. She had attributed these symptoms to a previous horseback riding incident.
- Regarding possible nonmotor symptoms, Patient 1 had long-term pain in her back, neck and shoulder and current complaints of increased urinary frequency and urgency. She did not have constipation. Patient 2 had been experiencing lower back pain for some years, and she also mentioned fatigue and altered feeling in her left foot. Constipation had been present since childhood and had not worsened since. Neither patient reported symptoms of rapid eye movement sleep behavior disorder.
- Neurological examination showed asymmetrical slight to mild rigidity and bradykinesia with an asymmetrically reduced arm swing in both patients. Patient 1 also had brisk tendon reflexes (without Babinski sign) on the right side, which was most affected by rigidity, and a subjective sensory deficit in the lower arm and lower leg on the same side. The arm swing was reduced on this side as well. Patient 2 also had slight postural instability and a slight rest tremor on the most symptomatic (left) side; in Patient 1, no tremor was present. Brain magnetic resonance imaging (MRI) was normal in both patients; in Patient 1, spinal cord pathology was also ruled out by MRI and nerve conduction studies, and electromyography ruled out peripheral neuropathy of the arm affected by sensory symptoms. DaT-SPECT showed a presynaptic nigrostriatal dopaminergic deficit in Patient 1; no such scan was performed in Patient 2. In both patients, dopaminergic medication (pramipexole and L-DOPA, respectively) improved symptoms. They were both diagnosed with idiopathic PD, and the interval between COVID-19 episodes and PD diagnoses was seven to ten months.
CASE DESCRIPTION
- COVID-19 unmasking Parkinson’s disease
- We describe two patients who were diagnosed with PD shortly after a COVID-19 episode. In both patients, careful history taking revealed motor and nonmotor symptoms that were already present before COVID-19. More specifically, in Patient 1, the symptoms now attributed to PD had already been present to a lesser degree two years before the infection. In Patient 2, there had been a deficient arm swing and subtle walking problems a year before COVID-19. Furthermore, both patients had a history of back pain as well as nonspecific sensory complaints on the side most affected by PD. Both pain [10] and sensory symptoms [11] are known nonmotor prodromal PD symptoms. Although these symptoms are common in the general population, the fact that the sensory symptoms lateralized to the side most affected by the akinetic-rigid syndrome makes an association with PD plausible. This suggests that, in our patients, COVID-19 unmasked underlying latent PD rather than causing it. This interpretation is further supported by the fact that the two patients did not have signs of hyposmia or hypogeusia, the presence of which would suggest neuroinvasion by SARS-CoV-2—although self-reporting of these symptoms may be unreliable [12].
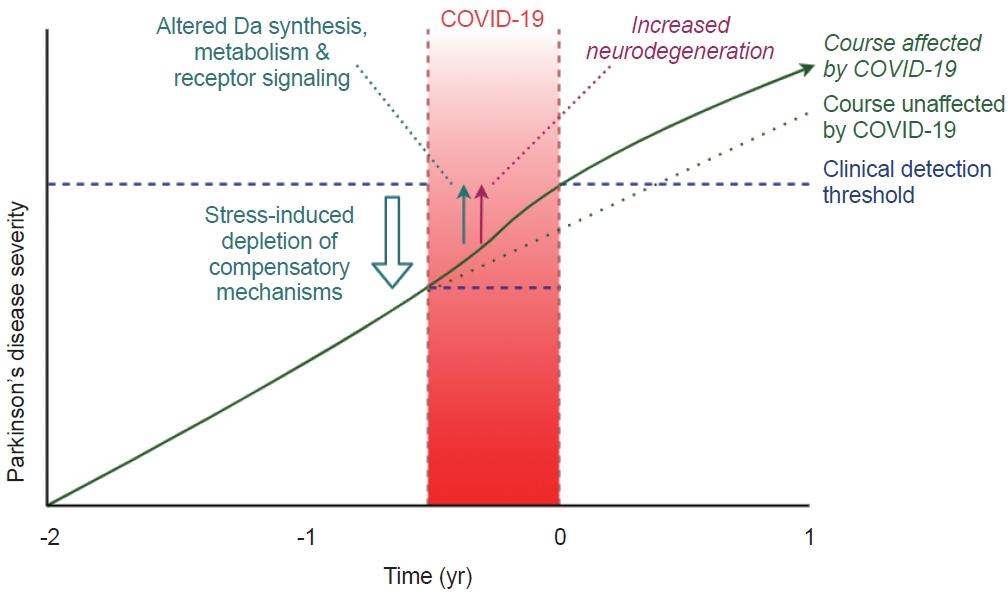
- Our observations raise the question of how COVID-19 may unmask latent PD. In symptomatic PD, it is well known that systemic infections can temporarily or even permanently worsen motor symptoms [13]. Indeed, in studies of PD patients with COVID-19, sixty percent experienced a worsening of their neurological symptoms [14]. This may result from the complex effects that a systemic infection has on the gut microbiome, the hormonal stress system, inflammation, and brain function. More specifically, inflammatory cytokines alter dopamine metabolism [15] and signaling [16]. The synthesis of dopamine may also be impaired. SARS-CoV-2 infection can lead to downregulation of ACE2 receptors, which (through coexpression and coregulation) might also lead to downregulation of the enzyme aromatic L-amino acid decarboxylase, which is a critical link in the biosynthesis of dopamine [17].
- Another contributing factor could be psychological stress. COVID-19 leads to psychological distress in PD patients [18], and stress can increase several PD motor symptoms [19]. Furthermore, animal studies have shown that stress can worsen or unmask symptoms in mice with dopamine-depleting brain lesions [20,21], possibly by depleting the reduced dopamine capacity to a level below the clinical threshold [20]. This explanation can be readily reconciled with the common recognition amongst movement disorders practitioners, that patients with newly-diagnosed PD typically associate the first manifestation of symptoms with a period of exaggerated stress in their lives.
- A third but very speculative hypothesis is that COVID-19 may accelerate ongoing neurodegeneration. Neuroinflammation in the substantia nigra has been mentioned as a possible precursor to neurodegeneration in PD [22,23]. Neuroinflammation may be increased in systemic infection [13,24], thereby possibly influencing neurodegeneration. Contributing mechanisms that have been proposed include SARS-CoV-2-induced gut dysbiosis24 and oxidative stress [25], which may potentiate neurodegeneration [13,24,26]. However, since such a process would presumably take significant time to manifest itself, it is unlikely that this mechanism explains cases of parkinsonism that emerge within days or weeks after a preceding COVID-19 infection. Additionally, direct evidence for COVID-19–induced neurodegeneration in humans is lacking. The current closest evidence is a nonhuman primate study in which both neuroinflammation and Lewy bodies could be demonstrated in the ventral midbrain of all SARS-CoV-2–infected rhesus macaques but not in healthy controls, which suggests that COVID-19 may induce neurodegeneration in primates [27].
- Last, SARS-CoV-2 infection has been proposed to act as a ‘second hit’ in individuals already ‘primed’ for developing PD, such as in the two patients with a genetic mutation [6].
- Taken together, these mechanisms suggest that the inflammatory response during COVID-19, together with possible ACE2-mediated effects and increased physiological and psychological stress, could lead to a worsening of nigrostriatal dopamine dysfunction, the depletion of compensatory mechanisms, or both (Figure 1). It remains unclear to what extent these effects are permanent.
- Sustained effects after the resolution of COVID-19 symptoms
- An intriguing question is why, in our patients, PD symptoms were not “remasked” after the infection was cleared and why systemic infection can result in sustained motor deterioration in PD patients [28]. This may suggest that changes have been set into motion that cannot be undone. It has been demonstrated that proteins associated with anti-inflammation and mitochondrial stress can remain elevated for months even after a mild SARS-CoV-2 infection [29], potentially paving the way for a sustained increase in the rate of neuroinflammation. Additionally, infection-induced altered dopamine metabolism and receptor signaling may not revert to baseline values after the infection is cleared. Indeed, a persisting need for higher L-DOPA doses compared to preinfectious doses has been reported in PD patients who had COVID-19 [30]. Finally, a temporary worsening of preexisting symptoms during an infection may have drawn the patients’ awareness to these complaints, causing them to seek attention from a neurologist. To disentangle these possibilities, long-term longitudinal studies are necessary.
- Suggestions for approaching post-COVID patients with signs of PD
- As a practical recommendation for the approach of post-COVID patients presenting with symptoms consistent with PD, we propose the following guidelines: 1) A careful history in search of evidence for (motor or nonmotor) prodromal symptoms already present before the COVID-19 episode should be performed; 2) clues for viral neuroinvasion, such as new-onset hyposmia, should be identified; 3) circumstances predisposing patients to unmasking, such as stress or a severe course of COVID-19, should be identified; and 4) characteristics suggesting a postinfectious central nervous system disorder should be identified.
- Our observations do not preclude the possibility of latent post-COVID parkinsonism presenting years after the infection. Time will tell whether such an entity will emerge.
DISCUSSION
-
Ethics Statement
The approval of an institutional review board or ethics committee was neither applicable nor sought. The described patients provided verbal informed consent for the publication of their cases. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
-
Conflicts of Interest
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
-
Funding Statement
None
-
Author Contributions
Conceptualization: Bastiaan R Bloem. Investigation: Milan Beckers, Rick C Helmich. Project administration: Milan Beckers. Supervision: Rick C Helmich. Visualization: Milan Beckers. Writing—original draft: Milan Beckers. Writing—review & editing: Bastiaan R Bloem, Rick C Helmich.
Notes
- The Radboudumc Centre of Expertise for Parkinson & Movement Disorders was supported by a center of excellence grant by the Parkinson’s Foundation.
Acknowledgments
- 1. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11:995–998.ArticlePubMedPDF
- 2. Brundin P, Nath A, Beckham JD. Is COVID-19 a perfect storm for Parkinson’s disease? Trends Neurosci 2020;43:931–933.ArticlePubMedPMC
- 3. Cohen ME, Eichel R, Steiner-Birmanns B, Janah A, Ioshpa M, Bar-Shalom R, et al. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol 2020;19:804–805.ArticlePubMedPMC
- 4. Faber I, Brandão PRP, Menegatti F, de Carvalho Bispo DD, Maluf FB, Cardoso F. Coronavirus disease 2019 and parkinsonism: a non-post-encephalitic case. Mov Disord 2020;35:1721–1722.ArticlePubMedPMCPDF
- 5. Makhoul K, Jankovic J. Parkinson’s disease after COVID-19. J Neurol Sci 2021;422:117331.ArticlePubMedPMC
- 6. Cavallieri F, Fioravanti V, Toschi G, Grisanti S, Napoli M, Moratti C, et al. COVID-19 and Parkinson’s disease: a casual association or a possible second hit in neurodegeneration? J Neurol 2021;269:59–61.ArticlePubMedPMCPDF
- 7. Méndez-Guerrero A, Laespada-García MI, Gómez-Grande A, Ruiz-Ortiz M, Blanco-Palmero VA, Azcarate-Diaz FJ, et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology 2020;95:e2109–e2118.ArticlePubMed
- 8. Morassi M, Palmerini F, Nici S, Magni E, Savelli G, Guerra UP, et al. SARS-CoV-2-related encephalitis with prominent parkinsonism: clinical and FDG-PET correlates in two patients. J Neurol 2021;268:3980–3987.ArticlePubMedPMCPDF
- 9. Merello M, Bhatia KP, Obeso JA. SARS-CoV-2 and the risk of Parkinson’s disease: facts and fantasy. Lancet Neurol 2021;20:94–95.ArticlePubMed
- 10. Beiske AG, Loge JH, Rønningen A, Svensson E. Pain in Parkinson’s disease: prevalence and characteristics. Pain 2009;141:173–177.ArticlePubMed
- 11. Müller B, Larsen JP, Wentzel-Larsen T, Skeie GO, Tysnes OB; Parkwest Study Group. Autonomic and sensory symptoms and signs in incident, untreated Parkinson’s disease: frequent but mild. Mov Disord 2011;26:65–72.ArticlePubMed
- 12. Beauchamp LC, Finkelstein DI, Bush AI, Evans AH, Barnham KJ. Parkinsonism as a third wave of the COVID-19 pandemic? J Parkinsons Dis 2020;10:1343–1353.ArticlePubMedPMC
- 13. Brugger F, Erro R, Balint B, Kägi G, Barone P, Bhatia KP. Why is there motor deterioration in Parkinson’s disease during systemic infections-a hypothetical view. NPJ Parkinsons Dis 2015;1:15014.ArticlePubMedPMCPDF
- 14. Kubota T, Kuroda N. Exacerbation of neurological symptoms and COVID-19 severity in patients with preexisting neurological disorders and COVID-19: a systematic review. Clin Neurol Neurosurg 2021;200:106349.ArticlePubMed
- 15. Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol 2012;33:315–327.ArticlePubMedPMC
- 16. Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, et al. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 2013;38:2179–2187.ArticlePubMedPMCPDF
- 17. Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID-19. J Med Virol 2020;92:1743–1744.ArticlePubMedPMCPDF
- 18. van der Heide A, Meinders MJ, Speckens AEM, Peerbolte TF, Bloem BR, Helmich RC. Stress and mindfulness in Parkinson’s disease: clinical effects and potential underlying mechanisms. Mov Disord 2021;36:64–70.ArticlePubMedPDF
- 19. Dirkx MF, Zach H, van Nuland AJ, Bloem BR, Toni I, Helmich RC. Cognitive load amplifies Parkinson’s tremor through excitatory network influences onto the thalamus. Brain 2020;143:1498–1511.ArticlePubMedPDF
- 20. Snyder AM, Stricker EM, Zigmond MJ. Stress-induced neurological impairments in an animal model of parkinsonism. Ann Neurol 1985;18:544–551.ArticlePubMed
- 21. Hemmerle AM, Dickerson JW, Herman JP, Seroogy KB. Stress exacerbates experimental Parkinson’s disease. Mol Psychiatry 2014;19:638–640.ArticlePubMedPDF
- 22. Galiano-Landeira J, Torra A, Vila M, Bové J. CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 2020;143:3717–3733.ArticlePubMedPDF
- 23. Olanow CW, Savolainen M, Chu Y, Halliday GM, Kordower JH. Temporal evolution of microglia and α-synuclein accumulation following foetal grafting in Parkinson’s disease. Brain 2019;142:1690–1700.ArticlePubMedPDF
- 24. Follmer C. Gut Microbiome imbalance and neuroinflammation: impact of COVID-19 on Parkinson’s disease. Mov Disord 2020;35:1495–1496.ArticlePubMedPMCPDF
- 25. Rosen B, Kurtishi A, Vazquez-Jimenez GR, Møller SG. The intersection of Parkinson’s disease, viral infections, and COVID-19. Mol Neurobiol 2021;58:4477–4486.ArticlePubMedPMCPDF
- 26. Dewanjee S, Vallamkondu J, Kalra RS, Puvvada N, Kandimalla R, Reddy PH. Emerging COVID-19 neurological manifestations: present outlook and potential neurological challenges in COVID-19 pandemic. Mol Neurobiol 2021;58:4694–4715.ArticlePubMedPMCPDF
- 27. Philippens IH, Böszörményi KP, Wubben JA, Fagrouch ZC, van Driel N, Mayenburg A, et al. SARS-CoV-2 causes brain inflammation and induces Lewy body formation in macaques. BioRxiv 432474 [Preprint]. 2021 [cited 2022 May 3]. Available at: https://doi.org/10.1101/2021.02.23.432474. Article
- 28. Umemura A, Oeda T, Tomita S, Hayashi R, Kohsaka M, Park K, et al. Delirium and high fever are associated with subacute motor deterioration in Parkinson disease: a nested case-control study. PLoS One 2014;9:e94944. ArticlePubMedPMC
- 29. Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid-19’ - The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients [version 2; peer review: 2 approved]. F1000Res 2021;9:1349.ArticlePMCPDF
- 30. Leta V, Rodríguez-Violante M, Abundes A, Rukavina K, Teo JT, Falup-Pecurariu C, et al. Parkinson’s disease and post-COVID-19 syndrome: the Parkinson’s long-COVID spectrum. Mov Disord 2021;36:1287–1289.PubMedPMC
REFERENCES
Figure & Data
References
Citations

- SARS-CoV-2 and Parkinson’s Disease: A Review of Where We Are Now
Iro Boura, Mubasher A. Qamar, Francesco Daddoveri, Valentina Leta, Karolina Poplawska-Domaszewicz, Cristian Falup-Pecurariu, K. Ray Chaudhuri
Biomedicines.2023; 11(9): 2524. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite