ABSTRACT
- Various neurologic manifestations of herpes simplex virus (HSV) encephalitis have been reported on the literatures. Chorea, ballism, choreoathetosis and myoclonus were reported as movement disorders which might be related with brain lesion by HSV encephalitis, but negative myoclonus (NM) has never been reported before. NM can be characterized as a shock-like involuntary jerky movement caused by a sudden, brief interruption of muscle activity. We experienced a case of HSV encephalitis with NM in unilateral arm and leg. In polygraphic monitoring, electroencephalography (EMG) silent periods are 50–250 ms in duration with no detectable EMG correlate.
-
Keywords: Herpes simplex virus; Encephalitis; Negative myoclonus; Asterixis
Various neurologic manifestations of herpes simplex virus (HSV) encephalitis have been reported on the literatures. Chorea,1 ballism,2 choreoathetosis3 and myoclonus4 were reported as movement disorders which might be related with brain lesion by HSV encephalitis, but negative myoclonus (NM) has never been reported before. NM can be characterized as a shock-like involuntary jerky movement caused by a sudden, brief interruption of muscle activity.5 We experienced a case of HSV encephalitis with NM in unilateral arm and leg.
Case
- A 56-year-old male patient visited emergency room of our hospital with myalgia, chill and fever of 38.0°C. He revealed intrahepatic ductal dilatation on the abdomen CT and positive antibody test for Clonorchis sinensis. He was admitted to the department of gastroenterology and took oral metronidazol. Initially he was alert, but on the next day he got drowsy. We conducted him brain MRI which showed bitemporal signal change with enhancement and edema more prominently on the right side (Figure 1A). He was transferred to our neurology department. On cerebrospinal fluid (CSF) study, white bood cell count was 8/mm3 (100% lymphocyte) with normal glucose (52 mg/dL) and increased protein (92 mg/dL). Electroencephalography (EEG) showed 5–7 Hz medium to high amplitude background slowing without epileptiform discharge. CSF HSV IgG and IgM antibodies were initially negative. CSF HSV type 1 polymerase chain reaction (PCR) was positive whereas CSF HSV type 2 PCR was negative. We diagnosed him as HSV type 1encephalitis. We managed him with intravenous acyclovir (30 mg/kg/day) for 3 weeks. Except for slight drowsiness and memory impairment, the other neurologic deficit was not found. About hospital day,30 he showed characteristics change with irritability, overeating, and inappropriately increased sexual desire. We considered these phenomena as Kluver-Busy syndrome and tried him escitalopram (10 mg/day), valproic acid (1,000 mg/day) and quetiapine (25 mg/day) and these medications had some effect. On hospital day,35 he was discharged from hospital with memory deficit and mild general weakness. After 10 days he was admitted our department again with delusion and visual hallucination. Through neurological examination, we found out that he got still memory impairment and newly developed mild weakness on his left limbs with NM (Video). On follow-up cerebrospinal fluid study, white bood cell count was 30/mm3 (100% lymphocyte) with normal glucose (42 mg/dL) and increased protein (80 mg/dL). CSF HSV IgG and IgM antibodies were all positive. CSF HSV type 1 PCR became negative. Follow-up brain MRI showed signal change on bilateral white matter and right thalamus and internal capsule and bilateral temporal cortical laminar necrosis (Figure 1B, C). Brain perfusion SPECT with technetium-99m ethyl cysteinate dimer [Tc-99m ethyl cysteinate dimer (ECD)] reveals perfusion decrease in the right fronto-parieto-temporal cortices, thalamus, and basal ganglia (Figure 1D). EEG showed brief or long runs of periodic isolated triphasic waves on right frontotemporal electrode. We changed the antiepileptic drug valproic acid to phenytoin.
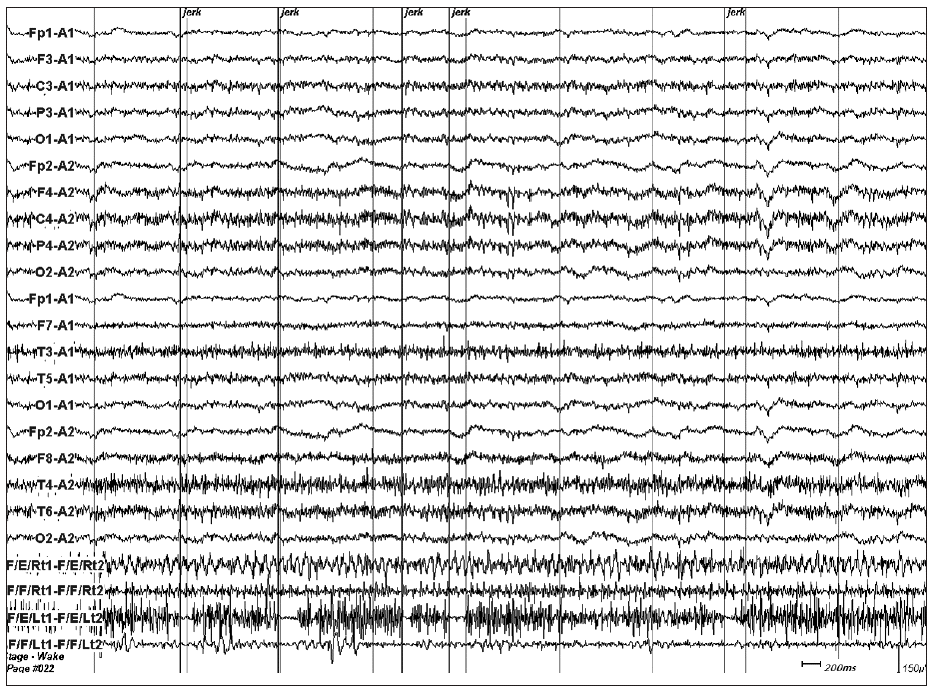
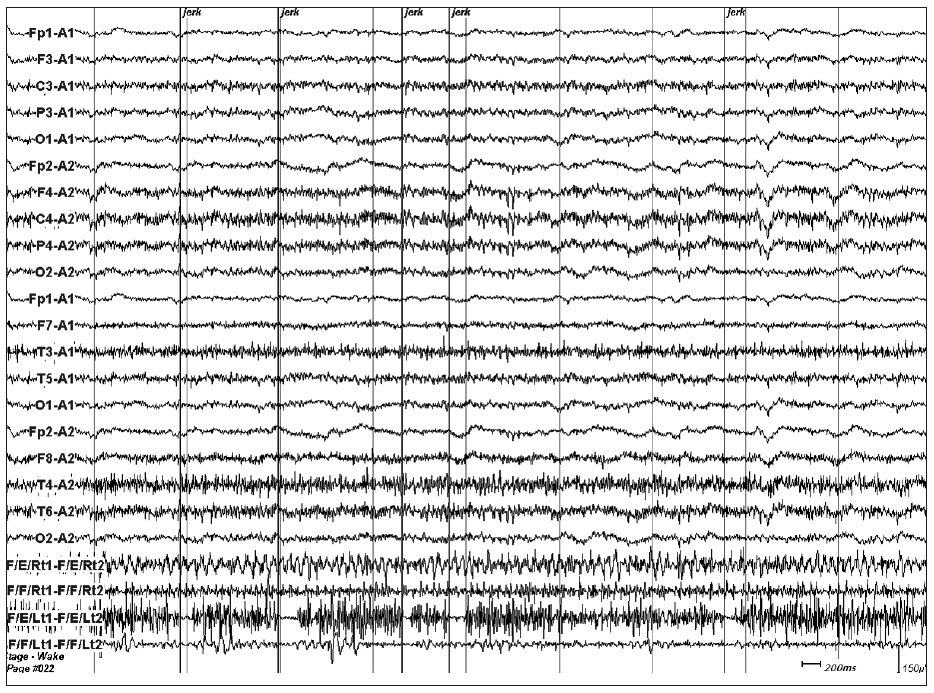
- And then we conducted EEG-electromyography (EMG) monitoring, which showed no cortical electrical change with myographic pauses that were related with negative myoclonic jerks (Figure 2). The duration of these electrical pauses ranged from 50 to 250 milliseconds (ms). We concluded that the cause of NM was due to subcortical brain damage such as right thalamic lesion and the right internal capsule lesion resulted in left hemiparesis. And We added clonazepam to phenytoin. His NM got better with improvement of weakness and discharged from hospital with constant memory impairment after two-month hospitalization.
Discussion
- Asterixis may be seen with metabolic encephalopathy including hepatic, renal and respiratory failure, drug intoxication, and electrolyte imbalance. In such cases the asterixis is usually bilateral, although often asymmetric. These features have led to the perception that asterixis represents a non-specific and non-localising abnormality of the motor system. In contrast to bilateral NM, unilateral cases are relatively uncommon and have been associated with a variety of focal cerebral lesions.6
- Previous study of stroke cases found 30 patients with NM out of 1,550 acute stroke patients and the lateral thalamus was the most frequently involved site with NM. But, other sites of brain such as basal ganglia, midbrain, pons and cerebellum were also involved.7 The pathogenic mechanism for NM remains unclear. The postural stability or tonic control of the extremities is related to multiple brainstem spinal pathways such as the vestibulospinal, reticulospinal, or rubrospinal tracts. These systems are regulated by supratentorial structures. The ventrolateral nucleus of the thalamus is the area in which cerebellar-rubral or vestibulocerebellar fibers converge, and is also connected with the prefrontal area.7 In our patient, the follow-up brain MRI showed the lesions in right thalamus and internal capsule. The medial thalamic lesion had been also present on the first brain MRI. So we supposed the pathophysiological cause of NM might be the lesion in the lateral thalamic region close to the internal capsule.
- HSV encephalitis is a morbid disease, with a 70% mortality rate in the absence of treatment and 10% with current antiviral therapy.1 Movement disorders related to HSV encephalitis are not commonly reported in the existing literature. Chorea, ballism, choreoathetosis and myoclonus have been infrequently cited in the literature as a clinical presentation of HSV encephalitis. The brain parenchymal damage due to HSV encephalitis may involve the thalamus or its pathways, the movement disorder may be observed. But, NM related to HSV encephalitis has never reported yet. This is probably attributed to the fact that not every patient was examined with the stretching of the arm in previous HSV encephalitis.
- In this case NM with the deterioration of brain lesions in the course of recovery might be caused by whether the relapse or natural course of HSV encephalitis. Relapse of HSV encephalitis occurs in up to 10%. In relapsing cases, there is clinical deterioration, neuropsychologic deficits, expansion of the lesions in MRI, and presence of viral replication or reactivation in the CSF proven by PCR for HSV type 1 DNA.8 We think in our case, the finding that the follow-up CSF HSV type 1 PCR became negative means it was not the relapse. A previous report showed MRI deterioration in HSV encephalitis despite clinical recovery.8 An immune-mediated process is suspected to be responsible for the MRI progressive changes. We suppose that clinical deterioration of this case might be correlated with severe brain MRI deterioration.
- NM can be classified on the basis of the possible site of its generator. NM of subcortical and cortical origin has been described. In cortical NM, EMG silent periods are usually longer in duration (100–400 ms) as compared to subcortical myoclonus. Subcortical NM is usually confirmed by the EMG studies which clearly demonstrated that the involuntary movements were synchronous with loss of muscle tonus and EMG silence without any detectable EEG correlate.5 In this case, EMG silent periods were 50–250 ms in duration and any detectable EEG correlate was not present. These findings were compatible with subcortical NM.
- In the literatures, treatment of subcortical NM is not elusive and limited by the rarity of its condition. Antiepileptic drugs such as carbamazepine, valproic acid, phenytoin, lamotrigine and oxcarbazepine have been reported to be able to induce or aggravate NM. On the contrary, levitracetam, valproic acid and ethosuximide have been reported to be able to improve NM.5 Initially we treated him with valproic acid for Kluver-Busy syndrome, then the patient got NM. This may explain that valpoic acid could elicit NM. But considering the unilaterallity of NM, controlateral thalamic lesion was more responsible for NM (allowing for valproic acid effect). Next visit, we treated him with phenytoin instead of valproic acid because of his first EEG finding of periodic isolated triphasic waves on right frontotemporal electrode, which could elicit cortical NM. We added clonazepam and his myoclonus got better with improvement of weakness. The improvement may be related with drug effect or the healing process of right thalamic lesion.
Legend to the Video
- The patient showed negative myoclonus on his left arm and leg. When he was instructed to raise his both legs at supine position, his left leg drifted down with arrhythmical and jerky movements which were associated with brief loss of hip flexor and knee extensor muscles tone. Because of his left leg weakness the examiner’s hands supported his both legs. And then when he outstretched his both arms with hand pronation at supine position, his left hand also showed slight drift with irregular and jerky movements which were associated with the loss of finger and wrist extensor muscles tone.
Notes
-
The authors have no financial conflicts of interest.
Figure 1A: Initial brain MRI showed hyperintensity with slight edema on the bilateral temporal and insular cortices and right thalamus on T2-weighted and FLAIR images. B: Follow-up brain MRI after 49 days revealed hyperintesity on the bilateral white matter and right thalamus with atrophic change at the bilateral temporal and insular cortices on T2-weighted imgages. C: Cortical laminar necrosis was observed in the bilateral temporal and insular cortices on the follow-up T1-weighted MRI. Tc-99m ECD brain SPECT performed when patient had negative myoclonus showed decreased perfusion on the right fronto-parieto-temporal cortices, thalamus, and basal ganglia.
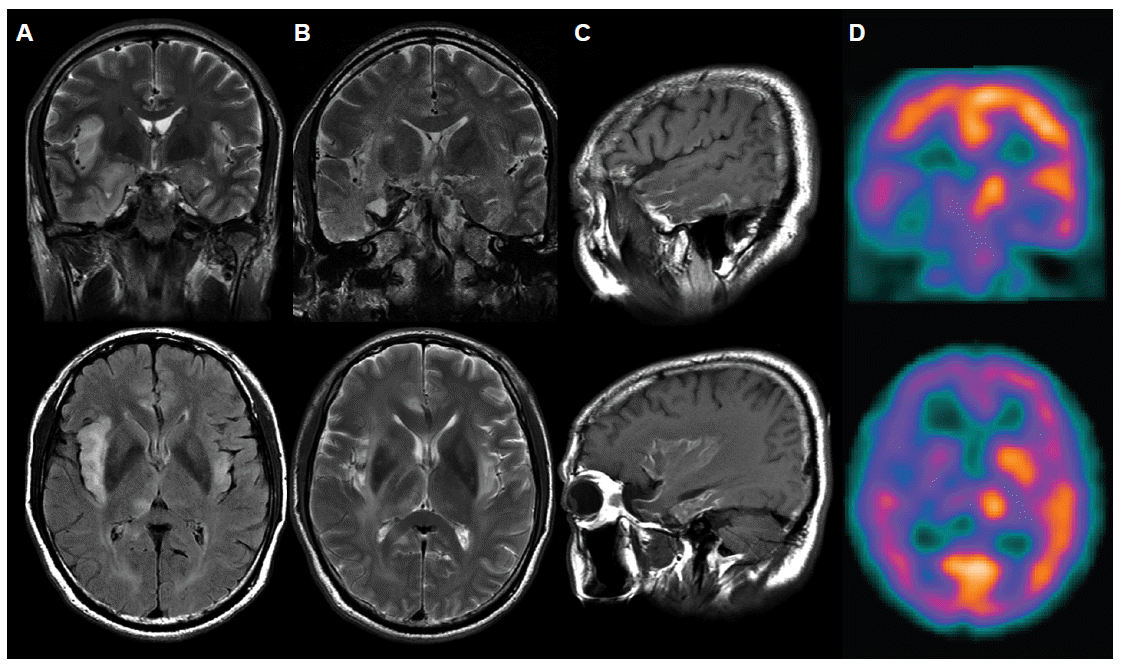
Figure 2EEG and EMG with surface electrodes on the bilateral forarm flexor and extensor muscles recorded while the patient assumed his both upper extremities upstretched on supine position showed several electrically silent periods on left forearm extensor muscle. These silent periods were related with NMs. The epoch comprised of 10 seconds. The last four channels are EMG recording from right forearm extensor, flexor, and left forearm extensor and flexor. EEG: electroencephalography, EMG: electroencephalography, NM: negative myoclonus.
REFERENCES
- 1. Gascon GG, al-Jarallah AA, Okamoto E, al Ahdal M, Kessie G, Frayha H. Chorea as a presentation of herpes simplex encephalitis relapse. Brain Dev 1993;15:178–181.ArticlePubMed
- 2. Baik JS, You SJ, Lee MS. Bilateral ballism after herpes encephalitis with thalamic lesion. Parkinsonism Relat Disord 2010;16:303–304.ArticlePubMed
- 3. Kullnat MW, Morse RP. Choreoathetosis after herpes simplex encephalitis with basal ganglia involvement on MRI. Pediatrics 2008;121:e1003–e1007.ArticlePubMed
- 4. Urushitani M, Wakita H, Ikeda A, Akiguchi I, Shibasaki H, Kimura J. Chronic herpes simplex encephalitis initially presenting with persistent myoclonus. Rinsho Shinkeigaku 1993;33:880–885.PubMed
- 5. Rubboli G, Tassinari CA. Negative myoclonus. An overview of its clinical features, pathophysiological mechanisms, and management. Neurophysiol Clin 2006;36:337–343.ArticlePubMed
- 6. Stell R, Davis S, Carroll WM. Unilateral asterixis due to a lesion of the ventrolateral thalamus. J Neurol Neurosurg Psychiatry 1994;57:116–118.ArticlePubMedPMC
- 7. Kim JS. Asterixis after unilateral stroke: lesion location of 30 patients. Neurology 2001;56:533–536.ArticlePubMed
- 8. Markoula S, Giannopoulos S, Pelidou SH, Argyropoulou M, Lagos G, Kyritsis AP. MRI deterioration in herpes simplex encephalitis despite clinical recovery. Neurologist 2009;15:223–226.ArticlePubMed
Citations
Citations to this article as recorded by

- Epileptic negative myoclonus in herpes simplex virus encephalitis
Neel Fotedar, James Dolbow, Madhura Layfield, Suraj Thyagaraj, Jonathan Zande
Epileptic Disorders.2022; 24(2): 417. CrossRef - Myoclonus in the critically ill: Diagnosis, management, and clinical impact
Raoul Sutter, Anette Ristic, Stephan Rüegg, Peter Fuhr
Clinical Neurophysiology.2016; 127(1): 67. CrossRef - Chorée aiguë après encéphalite herpétique : réplication virale ou mécanisme immunologique ?
H. Benrhouma, A. Nasri, I. Kraoua, H. Klaa, I. Turki, N. Gouider-Khouja
Archives de Pédiatrie.2015; 22(9): 961. CrossRef


 KMDS
KMDS
 E-submission
E-submission


 PubReader
PubReader ePub Link
ePub Link Cite
Cite