Focused Vibrotactile Stimulation with Cueing Effect on Freezing of Gait in Parkinson’s Disease: Two Case Reports
Article information
Abstract
Freezing of gait (FOG) is a common occurrence in patients with Parkinson’s disease (PD) that leads to significant limitations in mobility and increases risk of falls. Focused vibrotactile stimulation and cueing are two methods used to alleviate motor symptoms, including FOG, in patients with PD. While effective on their own, the effect of combining both focused vibrotactile stimulation and cueing has yet to be investigated. Two patients, both with a history of PD, suffered from frequent FOG episodes that failed to respond adequately to medication. A novel vibrotactile stimulation device that delivered rhythmic kinesthetic stimuli onto the sternum successfully reduced FOG episodes in both patients and drastically improved their mobility as measured by the Timed Up and Go test. We found that a combination of focused vibrotactile stimulation and cueing was effective in reducing FOG episodes in two patients with PD. Further well-designed prospective studies are needed to confirm our observations.
Freezing of gait (FOG) is a common occurrence in patients with advanced stages of Parkinson’s disease (PD) and greatly impairs their mobility, hence significantly increasing their risk of falls. Episodes of FOG tend to occur at the beginning of movement, upon turning or upon approaching an object.
Studies of vibrotactile stimulation in patients with PD showed significant improvement in motor tasks when vibratory stimulation was delivered to muscles of the extremities [1,2]. In addition, the use of external rhythmic cues to alleviate symptoms, including those of PD, has also been extensively studied [3]. Using external cues, also known as cueing, has been well established to be effective in alleviating gait symptoms, including FOG, in patients with PD.
In this case study, we present two patients with PD. Both showed drastic improvement in FOG episodes and mobility with the use of a novel device that has both vibrotactile stimulation and cueing effects.
CASE REPORT
Case 1
A 76-year-old man with a 9-year history of PD reported progressive worsening of his symptoms, with problems in motor function, stability, speech and concentration. The patient required a cane to mobilize and experienced frequent FOG episodes. He had a New Freezing of Gait Questionnaire (NFOG-Q) score of 24 [4]. He had no other significant neurological or medical history. His medication consisted of 10 mg/100 mg of carbidopa/levodopa four times a day and 10 mg of ropinirole once daily. Despite the gradual increase in the dosage of his medications, the patient felt that his medications could no longer provide adequate control of his symptoms.
The patient’s Timed Up and Go (TUG) time with device-off was 31.02 seconds. He experienced significant FOG episodes during the turning component of the test (Supplementary Video 1 [in the online-only Data Supplement] showing medication-on and device-off states). When the TUG test was repeated 30 minutes after turning on the device, the patient’s TUG time was 23.11 seconds, and he experienced no FOG episodes (Supplementary Video 1 [in the online-only Data Supplement] showing medication-on and device-on states). The patient reported feeling a positive difference with the use of the device and denied having any pain, discomfort or intrusion from using the device.
Case 2
A 75-year-old man with a 15-year history of PD suffered from deteriorating coordination, postural instability and FOG. He suffered from frequent falls, often daily, and was dependent on his wife’s assistance in mobilizing. He had the maximum NFOG-Q score of 28. His past medical history included hypotension and a sigmoid volvulus for which a colectomy was indicated. He had no other significant neurological history. His medication comprised 25 mg/100 mg of levodopa/benserazide taken twice a day, 12 mg of ropinirole taken once a day, 25 mg/100 mg of carbidopa/levodopa taken five times a day and 5 mg of subcutaneous apomorphine taken as needed.
The patient’s TUG time with the device off was 301.22 seconds, and he required his wife’s assistance for additional stability and visual guidance on where to step. The patient had difficulty rising from a chair and subsequently experienced multiple significant FOG episodes that were worse upon turning (Supplementary Video 2 [in the online-only Data Supplement] showing medication-on and device-off states). The TUG test was repeated 30 minutes after turning on the device. Despite not adhering to the protocols of the TUG test, the patient was able to complete the test independently, with improved stability and significantly fewer FOG episodes (Supplementary Video 2 [in the online-only Data Supplement] showing medication-on and device-on states). Of note, the time taken for him to complete two laps was 45.02 seconds.
DISCUSSION
Current treatment for PD largely involves restoring dopaminergic tone in the basal ganglia by administering levodopa. While effective at improving the classical symptoms of PD, levodopa’s effect on FOG remains limited [4,5]. Emerging evidence has shown that FOG can be improved with deep brain stimulation (DBS) of the subthalamic nucleus [6]. However, trial evidence remains mixed, and DBS requires an invasive surgical intervention with life-threatening risks, including hemorrhage and infection, and is largely only available to patients with severe, refractory disease [7].
The intervention is a noninvasive and wearable device for patients with PD that delivers rhythmic kinesthetic stimuli through a specialized frequency and pattern onto the sternum. The device is small and lightweight, measuring 40 mm in diameter, 11 mm in height, and 17 g in weight, and is attached to the sternum via medical adhesive patches. The device houses a motor that produces not only vibrotactile stimulation but also a cueing effect when placed on the patient’s sternum (Figure 1).
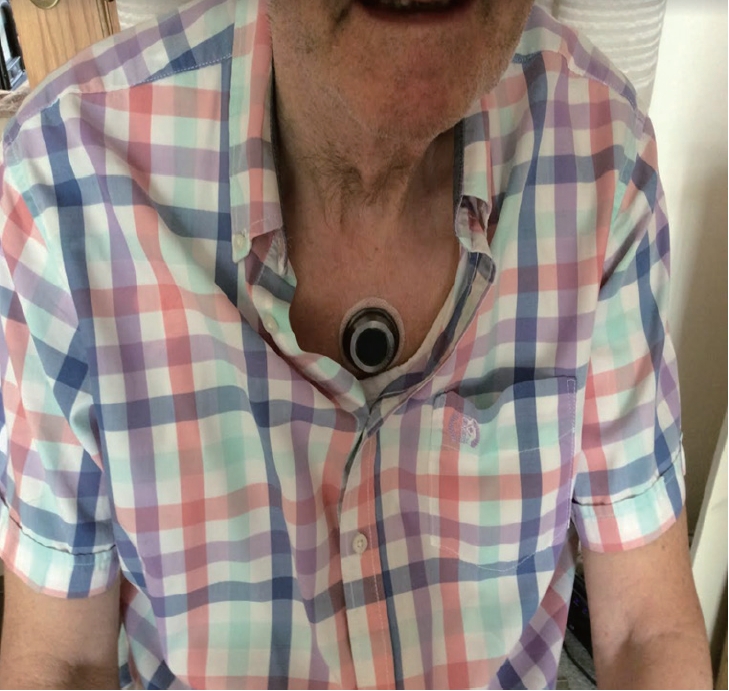
The novel vibrotactile stimulation device (CUE1, Charco Neurotech Ltd., London, United Kingdom) is attached to the sternum.
Focused vibrotactile stimulation activates muscle spindles, and the proprioceptive signals that are generated are relayed to the brain via type IA afferent nerve fibers [8]. This produces the kinesthetic illusion that trunk muscles are moving in the absence of any activity in the muscles themselves. In addition, focused vibrotactile stimulation has been shown to improve the performance of motor tasks in patients with PD [1].
Notably, in the TUG test, the patient in Case 2 exhibited a “marching gait” in the device-on state, whereas he could not march in the device-off state despite his wife’s guidance. This phenomenon is considered a reflection of the pulsatile stimulation of the device. Cueing is extensively used in improving gait symptoms in patients with PD and is achieved by bypassing the internal rhythm deficit of the basal ganglia [9]. The defective basal ganglia-supplementary motor area (SMA) circuit is bypassed using alternative circuitry that enables movement preparation and initiation to take place [10]. Somatosensory cues using vibrotactile stimulation devices stimulate proprioceptive inputs, which help provide enhanced information on limb position and movement, both of which are believed to be deficient in patients with PD.
The two case reports have highlighted the benefits of combining both focused vibrotactile stimulation and cueing in providing a noninvasive treatment of PD symptoms using the novel vibrotactile stimulation device. Further research is needed to validate these findings with a larger sample size, standardization of testing location, and the use of gait analysis with other objective measurements.
Supplementary Material
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.21076.
Video 1.
The patient in Case 1 shows a considerable improvement in gait, including reduced freezing of gait in the device-on state compared with device-off state.
Video 2.
The patient in Case 2 reveals a severe degree of gait disturbances, including freezing of gait and postural instability in the device-off state. However, the patient shows amelioration of his gait disturbances in the device-on state. The patient displays a “marching gait” in the device-on state, indicating a response to the cueing effect.
Notes
Ethics Statement
All procedures were performed in accordance with the ethical standards of the institution and/or the National Research Committee, as well as with the 1964 Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patients for the publication of this case report and any accompanying images.
Conflicts of Interest
Tan XS, Pierres F, Dallman-Porter A, and Hardie-Brown W are members of the medical research team in Charco Neurotech Ltd., London, United Kingdom. Dr. Kwon serves as a scientific advisor coresearcher in Charco Neurotech Ltd., London, United Kingdom.
Funding Statement
This work was supported by the Soonchunhyang University Research Fund (No. 20200501).
Author Contributions
Conceptualization: Kyum-Yil Kwon. Data curation: all authors. Formal analysis: all authors. Funding acquisition: Kyum-Yil Kwon. Investigation: Kyum-Yil Kwon. Methodology: Kyum-Yil Kwon. Supervision: Kyum-Yil Kwon. Writing—original draft: Xiu Sheng Tan, Floyd Pierres, Kyum-Yil Kwon. Writing—review & editing: all authors.
Acknowledgements
We would like to thank the two participants and their families for consenting to and supporting this publication.
