Articles
- Page Path
- HOME > J Mov Disord > Volume 4(1); 2011 > Article
-
Original Article
The Sequence Effect inDe Novo Parkinson’s Disease - Suk Yun Kanga,b, Toshiaki Wasakaa, Ejaz A. Shamima,c, Sungyoung Auhd, Yoshino Uekia, Nguyet Danga, Mark Halletta
-
Journal of Movement Disorders 2011;4(1):38-40.
DOI: https://doi.org/10.14802/jmd.11006
Published online: April 30, 2011
aHuman Motor Control Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA
bDepartment of Neurology, Medical Center, Hallym University College of Medicine, Seoul, Korea
cKaiser Permanente Midatlantic Permanente Medical Group, Suitland, MD, USA
dClinical Neurosciences Program, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA
- Corresponding author: Mark Hallett, MD, Human Motor Control Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, 10 Center Drive MSC 1428, Bldg. 10, Room 7D37, Bethesda, MD 20892-1428, USA, Tel +1-301-496-9526, Fax +1-301-480-2286, E-mail hallettm@ninds.nih.gov
Copyright © 2011 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 12,992 Views
- 57 Download
- 12 Crossref
ABSTRACT
-
Background and Purpose
- The sequence effect (SE) in Parkinson’s disease (PD) denotes progressive slowness in speed or progressive decrease in amplitude of repetitive movements. It is a well-known feature of bradykinesia and is considered unique in PD. Until now, it was well-documented in advanced PD, but not in drug-naïve PD. The aim of this study is to know whether the SE can also be measured in drug-naïve PD.
-
Methods
- We measured the SE with a computer-based, modified Purdue pegboard in 4 drug-naïve PD patients, which matched our previous study with advanced PD patients.
-
Results
- We observed progressive slowness during movement, that is, SE. Statistical analysis showed a strong statistical trend toward the SE with the right hand, but no significance with the left hand. There was no statistical significance of SE with either the more or less affected hands.
-
Conclusions
- These results indicate that the SE can be identified in drug-naïve PD, as well as in advanced PD, with objective measurements and support the idea that the SE is a feature in PD observed during the early stage of the disease without medication.
- Subjects
- We collected the complete data of four patients (1 woman, 3 men). All patients were right-handed. Their mean (± SD) age was 64.3 ± 9.3 years. The mean (± SD) disease duration was 2.6 ± 1.7 years. Hoehn and Yahr stages were 2. Mini-Mental State Examination (29.3 ± 0.5), Hamilton Depression Rating Scale (3 ± 2), Fatigue Severity Scale (4.2 ± 1.1), and Multidimensional Fatigue Inventory (57.8 ± 15.0) were evaluated (Table 1). We recruited patients from the National Institute of Neurological Disorders and Stroke (NINDS) Clinics. All patients gave written informed consent for this study protocol approved by the NINDS Institutional Review Board.
- Procedures
- The experimental details and analysis of the SE were the same as in the previous study.3 We assessed the SE as a progressive lengthening of peg movement time for successive peg movements, using a Modified Purdue Pegboard Test and a computer-based device (part of the At-Home Testing Device, Intel, courtesy of the Kinetics Foundation).9 The Pegboard Test had a vertical line of eight holes on both the right and left sides. The task started on the right side. We asked patients to move individual pegs from the right to the left side as quickly as possible. That constituted one run. The device could store the time of pulling-out and pushing-in of each peg. There were six runs, three with the right hand first, followed by three with the left hand. There was a 10-second pause between runs and each run began with a beep.
- Data and statistical analysis
- To assess the SE, we calculated differences between the times to move the first four pegs and the last four pegs for each hand. We did not calculate either the second run with the right hand or the fifth run with the left hand because the direction was opposite to the other two runs for each hand and we thought that the opposite direction might bias the data. We averaged the differences over the two runs, per hand, pegboard test, and patient. Patients were asked to visit four times and to repeat the pegboard test during each visit. Thus, we collected four sets of data. To know whether the SE in both hands was statistically significant, differences were averaged across the four visits for each hand (right, left, more affected, and less affected, respectively) and evaluated using a Wilcoxon signed rank test.
Methods
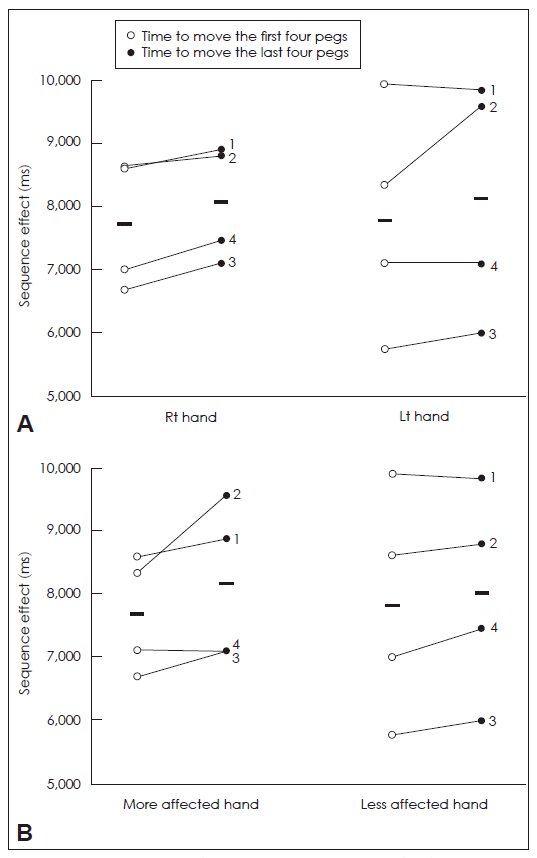
- There was progressive slowing (SE) during movement of the last four pegs. A Wilcoxon signed rank test showed a strong statistical trend toward the SE with the right hand, but no significance with the left hand (right hand, 7745.3 ± 513.7 ms. vs. 8082.8 ± 455.7 ms, p = 0.068; left hand, 7797.3 ± 887.8 ms. vs. 8144.8 ± 937.5 ms, p = 0.465) (Figure 1A). A Wilcoxon signed rank test did not show statistically significant SE with either the more or less affected hands (more affected hand, 7700.0 ± 465.0 ms. vs. 8186.3 ± 629.8 ms, p = 0.144; less affected hand, 7842.5 ± 912.7 ms. vs. 8041.3 ± 828.9 ms, p = 0.144) (Figure 1B).
Results
- These results indicate that the SE can be identified in drug-naïve PD, as well as in advanced PD. Additionally, the data show that the SE is a feature in PD and is observed during the early-stage of the disease without medication.
- The SE is well-known in PD.1,4,10,11 Patients with PD were especially slow while they performed complex or repetitive movements.2,11 The patients needed considerably more time to perform one task and exhibited a longer pause between one task and the next, compared with healthy volunteers.2,11 Patients with basal ganglia disorders also showed these abnormalities, but only PD patients needed progressively increased time to complete individual movements during repetitive movements.1 That is, the SE was observed in only PD patients.
- To date, the SE has only been measured in advanced PD with medication. In clinical practice, we can observe the SE from several types of repetitive movements such as finger tapping, writing, and gait in early, drug-naïve as well as advanced PD. Thus, one can assume that the SE would be measured during early, drug-naïve PD.
- It might be asked why the SE should be measured separately from other motor symptoms. It appears that the cause of all motor symptoms is not the same and dopaminergic medication does not improve all such symptoms.12–14 The clinical significance of the SE remains to be investigated. It has been suggested that it contributes to freezing of gait in PD.15 It was also postulated that the SE may be related to cognition3,16 and fatigue.1
- There are some limitations in this study. First, the sample size was small. The mean value of the SE was higher in the more affected hand than in the less affected hand, but there was no statistical significance. Second, we did not provide data from healthy volunteers; but because the SE has been demonstrated in various types of sequential movements in PD, and not in healthy volunteers,11 it is less likely that the healthy volunteers would show the same SE.
Discussion
- This work was supported by the Intramural Research Program of the NINDS at the NIH and the American Parkinson Disease Association. We appreciate the Kinetics Foundation (Ken Kubota, BS) and Intel Inc. (William De-Leeuw, BSEE and David Wolff, BS) for providing the at-home testing device and technical support. We thank Devera G. Schoenberg, MSc, for skillful editing.
Acknowledgments
Acknowledgments

| No. | Age (yr) | Sex | Duration (yr)* | H & Y | MMSE | UPDRS | HDRS | FSS | MFI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | F | 1.4 | 2 | 29 | 31 | 2 | 4.67 | 51 |
| 2 | 76 | M | 2 | 2 | 30 | 24 | 6 | 5.2 | 80 |
| 3 | 59 | M | 1.4 | 2 | 29 | 17 | 2 | 2.56 | 47 |
| 4 | 67 | M | 5 | 2 | 29 | 16 | 2 | 4.4 | 53 |
- 1. Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain 1992;115:1481–1495.ArticlePubMed
- 2. Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain 1987;110:361–379.ArticlePubMed
- 3. Kang SY, Wasaka T, Shamim EA, Auh S, Ueki Y, Lopez GJ, et al. Characteristics of the sequence effect in Parkinson’s disease. Mov Disord 2010;25:2148–2155.ArticlePubMedPMC
- 4. Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord 2006;21:1419–1424.ArticlePubMed
- 5. Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naïve Parkinson’s disease. Hum Brain Mapp Epub 2010;31:1928–1941.Article
- 6. Stoffers D, Bosboom JL, Deijen JB, Wolters Ech, Stam CJ, Berendse HW. Increased cortico-cortical functional connectivity in early-stage Parkinson’s disease: an MEG study. NeuroImage 2008;41:212–222.ArticlePubMed
- 7. Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain 2009;132:309–318.ArticlePubMed
- 8. Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, et al. Altered plasticity of the human motor cortex in Parkinson’s disease. Ann Neurol 2006;59:60–71.ArticlePubMed
- 9. Goetz CG, Stebbins GT, Wolff D, DeLeeuw W, Bronte-Stewart H, Elble R, et al. Testing objective measures of motor impairment in early Parkinson’s disease: feasibility study of an at-home testing device. Mov Disord 2009;24:551–556.ArticlePubMedPMC
- 10. Agostino R, Berardelli A, Formica A, Stocchi F, Accornero N, Manfredi M. Analysis of repetitive and nonrepetitive sequential arm movements in patients with Parkinson’s disease. Mov Disord 1994;9:311–314.ArticlePubMed
- 11. Berardelli A, Accornero N, Argenta M, Meco G, Manfredi M. Fast complex arm movements in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1986;49:1146–1149.ArticlePubMedPMC
- 12. Benice TS, Lou JS, Eaton R, Nutt J. Hand coordination as a quantitative measure of motor abnormality and therapeutic response in Parkinson’s disease. Clin Neurophysiol 2007;118:1776–1784.ArticlePubMed
- 13. Melvin KG, Doan J, Pellis SM, Brown L, Whishaw IQ, Suchowersky O. Pallidal deep brain stimulation and L-dopa do not improve qualitative aspects of skilled reaching in Parkinson’s disease. Behav Brain Res 2005;160:188–194.ArticlePubMed
- 14. Ferraye MU, Debȗ B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain 2010;133:205–214.ArticlePubMed
- 15. Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain 2009;132:2151–2160.ArticlePubMed
- 16. Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 2001;124:2131–2146.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Bradykinesia in Neurodegenerative Disorders: A Blinded Video Analysis of Pathology‐Proven Cases
Luca Marsili, Kevin R. Duque, Nathan Gregor, Elhusseini Abdelghany, Jesus Abanto, Andrew P. Duker, Matthew C. Hagen, Alberto J. Espay, Matteo Bologna
Movement Disorders.2023; 38(3): 496. CrossRef - The Sequence Effect Worsens Over Time in Parkinson’s Disease and Responds to Open and Closed-Loop Subthalamic Nucleus Deep Brain Stimulation
Yasmine M. Kehnemouyi, Matthew N. Petrucci, Kevin B. Wilkins, Jillian A. Melbourne, Helen M. Bronte-Stewart
Journal of Parkinson's Disease.2023; 13(4): 537. CrossRef - Neurofeedback-guided kinesthetic motor imagery training in Parkinson’s disease: Randomized trial
Sule Tinaz, Serageldin Kamel, Sai S. Aravala, Mohamed Elfil, Ahmed Bayoumi, Amar Patel, Dustin Scheinost, Rajita Sinha, Michelle Hampson
NeuroImage: Clinical.2022; 34: 102980. CrossRef - The Pathophysiological Correlates of Parkinson's Disease Clinical Subtypes
Daniele Belvisi, Andrea Fabbrini, Maria Ilenia De Bartolo, Matteo Costanzo, Nicoletta Manzo, Giovanni Fabbrini, Giovanni Defazio, Antonella Conte, Alfredo Berardelli
Movement Disorders.2021; 36(2): 370. CrossRef - The Effects of Intensive Neurorehabilitation on Sequence Effect in Parkinson's Disease Patients With and Without Freezing of Gait
Alessia Putortì, Michele Corrado, Micol Avenali, Daniele Martinelli, Marta Allena, Silvano Cristina, Valentina Grillo, Luca Martinis, Stefano Tamburin, Mariano Serrao, Antonio Pisani, Cristina Tassorelli, Roberto De Icco
Frontiers in Neurology.2021;[Epub] CrossRef - Evolving concepts on bradykinesia
Matteo Bologna, Giulia Paparella, Alfonso Fasano, Mark Hallett, Alfredo Berardelli
Brain.2020; 143(3): 727. CrossRef - Effectiveness of Exercise on the Sequence Effect in Parkinson’s Disease
Suk Yun Kang, Young Ho Sohn
Journal of Movement Disorders.2020; 13(3): 213. CrossRef - Neurophysiological correlates of bradykinesia in Parkinson’s disease
Matteo Bologna, Andrea Guerra, Giulia Paparella, Laura Giordo, Danilo Alunni Fegatelli, Anna Rita Vestri, John C Rothwell, Alfredo Berardelli
Brain.2018; 141(8): 2432. CrossRef - Insula as the Interface Between Body Awareness and Movement: A Neurofeedback-Guided Kinesthetic Motor Imagery Study in Parkinson’s Disease
Sule Tinaz, Kiran Para, Ana Vives-Rodriguez, Valeria Martinez-Kaigi, Keerthana Nalamada, Mine Sezgin, Dustin Scheinost, Michelle Hampson, Elan D. Louis, R. Todd Constable
Frontiers in Human Neuroscience.2018;[Epub] CrossRef - Sequence Effect in Parkinson’s Disease Is Related to Motor Energetic Cost
Sule Tinaz, Ajay S. Pillai, Mark Hallett
Frontiers in Neurology.2016;[Epub] CrossRef - Bradykinesia in early and advanced Parkinson's disease
Matteo Bologna, Giorgio Leodori, Paola Stirpe, Giulia Paparella, Donato Colella, Daniele Belvisi, Alfonso Fasano, Giovanni Fabbrini, Alfredo Berardelli
Journal of the Neurological Sciences.2016; 369: 286. CrossRef - Neural correlates of progressive reduction of bradykinesia in de novo Parkinson's disease
Eeksung Lee, Ji Eun Lee, Kwangsun Yoo, Jin Yong Hong, Jungsu Oh, Mun Kyung Sunwoo, Jae Seung Kim, Yong Jeong, Phil Hyu Lee, Young Ho Sohn, Suk Yun Kang
Parkinsonism & Related Disorders.2014;[Epub] CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite