Although the perioperative period of deep brain stimulation (DBS) treatment is undoubtedly pivotal for immediate therapeutic success, long-term treatment efficacy and reliability ultimately depend on the durability, functionality and (bio)compatibility of all implanted components. While routine monitoring generally helps prevent or timely correct potential functionality and durability issues, biocompatibility issues can be challenging to appropriately and timely diagnose and manage.
We present a rare case of a noninfectious tissue response manifesting as a green gelatinous mass on a battery casing encountered during routine battery replacement surgery.
A 70-year-old male patient with Parkinson’s disease had received bilateral subthalamic DBS in 2007, connected to two internal pulse generators (IPGs). Both IPGs had been previously replaced during an uneventful routine IPG replacement procedure in 2010. The patient was a research participant in the Queensland Parkinson’s Project (HREC approval 2011/730) and had signed a consent form for participation. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research ethics committee and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards.
In 2013, the patient suffered a fall, resulting in several fractured ribs on the right side and pleural effusion. Testing showed that the functional integrity of both IPGs was maintained. In May 2014, the patient presented with swelling over the right IPG without pain or skin reaction. Blood analysis demonstrated a normal white blood cell count and erythrocyte sedimentation rate but a mildly elevated CRP level (19 mg/L). The swelling was managed with a broad-spectrum antibiotic.
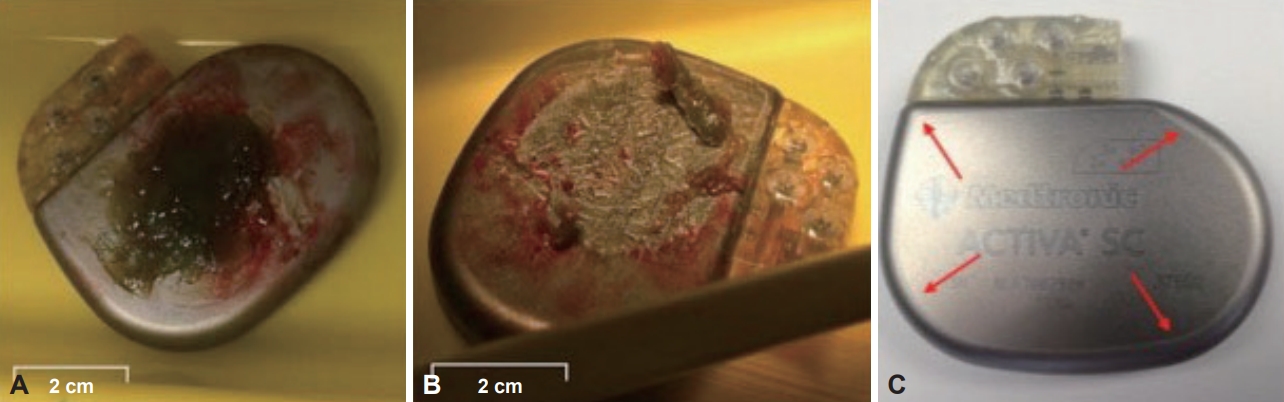
In July 2014, the patient was admitted to the hospital for routine IPG replacement surgery. The left IPG was unremarkable upon removal and was replaced successfully. Upon removal of the right IPG, an unusual pale green gelatinous mass on the outer casing of the IPG was observed (Figure 1A and B). The volume of the mass (36 × 22 × 5 mm) covered the logo-engraved surface. There were no clinical indications suggestive of infection at the time of surgery, and the patient had no prior concurrent medical indications other than Parkinson’s disease.
The IPG (Activa SC, Medtronic, Dublin, Ireland) was implanted 4 years earlier in the right infraclavicular subcutaneous pocket during a similar routine surgery and had functioned flawlessly until recent depletion. There were no immediate signs of damage (Figure 1A and B), and there were no indications of an abnormal lifespan. Normal impedance levels reflected the integrity of the device. The IPG was examined by the manufacturer, who reported no abnormalities, punctures or leaks. The patient was managed with a combination of carbidopa/levodopa/entacapone (75 mg/3 hrs; Stalevo, Orion Corporation, Espoo, Finland), trihexyphenidyl (5 mg, 4x/day; American) Cyanamid Company, Pearl River, NY, USA) and pramipexole dihydrochloride monohydrate (1.5 mg, 1x/day; Sifrol ER, Boehringer-Ingelheim, Ingelheim am Rhein, Germany) supplementary to DBS therapy.
The histopathology of the mass showed inspissated eosinophilic fibrinoid material with streaks of degenerate neutrophils within the center. No fungal elements were observed under direct microscopy. A fluorescent/Ziehl-Neelsen stain excluded acid-fast bacilli. Both gram staining of the green mass and a chest swab showed a few leucocytes but no microorganisms. Aerobic and anaerobic cultures showed no growth after 48 hours of incubation.
A new IPG (Activa SC) was implanted at the same site after the pocket was cleaned. The wound healed normally, and to date (2020), the patient has remained free of symptoms.
Although implanted biomaterials used in present-day surgical interventions are commonly well tolerated, no biomaterial is completely inert, leaving patients at risk for immediate or delayed complications. Immediate consequences can include temporary interruption or complete cessation of treatment due to replacement or complete removal of the implanted medical device.
Titanium has renowned biocompatibility due to a passivation layer formed by oxidative processes that occur instantly when exposed to biological fluids [1]. The passivation layer generally prevents further corrosive reactions; however, mechanical wear or damage influences the biocompatibility and potentially initiates the release of titanium ions, which can result in delayed physiological responses [2]. The outer casing of the recovered IPG was pure titanium with an insulating parylene coating confined to the back and sides, leaving an area of titanium exposed at the front (Figure 1C) [3]. The green mass had accumulated exclusively on the exposed titanium.
It is uncertain whether the two incidents are related, but it can be speculated that the fall in 2013 was implicated in the origin of the swelling in the following months and the subsequent discovery of the green mass in 2014. The fall may have resulted in a hematoma in the IPG pocket, but it can be expected this would have resolved well before the IPG surgery. Additionally, post-hematoma staining due to hemaglobin degradation products is not typically green.
Another possibility is that the passivation layer of the IPG case was sufficiently damaged as a result of the fall, leading to the release of titanium ions, provoking a reaction, and resulting in swelling. However, this leaves the pale green color of the mass unexplained, as tissue discoloration due to titanium ions is usually black or dark brown [4].
A more remote explanation could be exposure to a different metal altogether. It is not uncommon for impurities, including nickel, chromium, copper or chromate, to remain embedded in IPGs after production [5]. A fall is the most likely trigger for exposure to any suspected allergen. Damage to the passivation layer may have exposed traces of embedded metallic impurities, resulting in a physical response with subsequent green discoloration. Copper exposure, for instance, has been linked to green discoloration [6].
Although contamination during the initial implantation surgery could be considered, the four-year delay in the reaction resulting in the green mass suggests that this is unlikely. Whether the green mass was attributable to a tissue response to titanium or other metals remains unclear. Considering that the patient has had permanently implanted devices for more than eight years leading up to this event, it is unlikely that the reaction was systemic but rather points towards a local event. The patient has been carefully monitored and, to date (2020), has remained free of allergic or inflammatory symptoms.
Notes
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Author Contributions
Conceptualization: all authors. Data curation: Peter C. Poortvliet. Formal analysis: Peter C. Poortvliet. Funding acquisition: Peter C. Poortvliet, George Mellick. Investigation: Peter C. Poortvliet, Terry Coyne, Peter Silburn. Methodology: Peter C. Poortvliet. Project administration: Peter C. Poortvliet. Resources: all authors. Supervision: George Mellick, Peter Silburn. Validation: Peter C. Poortvliet, Terry Coyne, Peter Silburn. Visualization: Peter C. Poortvliet. Writing—original draft: Peter C. Poortvliet. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Acknowledgments
- This study was not funded, but the research was supported by Griffith University.
Figure 1.A: The explanted IPG with the green gelatinous mass attached to the outward facing, logo-engraved surface of the titanium casing. B: The IPG with the green gelatinous mass removed. The exposed IPG surface showed no immediate signs of damage. C: An IPG (Activa SC, Medtronic) showing the edge of the parylene film, leaving an area of titanium exposed at the front. IPG: internal pulse generator.

REFERENCES
- 1. Peters MS, Schroeter AL, van Hale HM, Broadbent JC. Pacemaker contact sensitivity. Contact Dermatitis 1984;11:214–218.ArticlePubMed
- 2. Forte G, Petrucci F, Bocca B. Metal allergens of growing significance: epidemiology, immunotoxicology, strategies for testing and prevention. Inflamm Allergy Drug Targets 2008;7:145–162.ArticlePubMed
- 3. Thyssen JP, Linneberg A, Menné T, Johansen JD. The epidemiology of contact allergy in the general population--prevalence and main findings. Contact Dermatitis 2007;57:287–299.ArticlePubMed
- 4. Park JY, Shin DH, Choi JS, Kim KH. Metallic discoloration on the right shin caused by titanium alloy prostheses in a patient with right total knee replacement. Ann Dermatol 2013;25:356–359.ArticlePubMedPMC
- 5. Bernard S, Baeck M, Tennstedt D, Haufroid V, Dekeuleneer V. Chromate or titanium allergy--the role of impurities? Contact Dermatitis 2013;68:191–192.ArticlePubMed
- 6. Peterson J, Shook BA, Wells MJ, Rodriguez M. Cupric keratosis: green seborrheic keratoses secondary to external copper exposure. Cutis 2006;77:39–41.PubMed
Citations
Citations to this article as recorded by

- Do Antibiotic-Impregnated Envelopes Prevent Deep Brain Stimulation Implantable Pulse Generator Infections? A Prospective Cohort Study
Michael Colditz, Tomas Heard, Peter Silburn, Terry Coyne
Stereotactic and Functional Neurosurgery.2024; : 1. CrossRef
 , Terry Coyne2, Peter Silburn2
, Terry Coyne2, Peter Silburn2 

 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite