ABSTRACT
- The aim of this article is to describe the 2017 revised consensus criteria for the clinical diagnosis of dementia with Lewy bodies (DLB) with future directions for the diagnostic criteria. The criteria for the clinical diagnosis of probable and possible DLB were first published as the first consensus report in 1996 and were revised in the third consensus report in 2005. After discussion at the International DLB Conference in Fort Lauderdale, Florida, USA, in 2015, the International DLB Consortium published the fourth consensus report including the revised consensus criteria in 2017. The 2017 revised criteria clearly distinguish between clinical features and diagnostic biomarkers. Significant new information about previously reported aspects of DLB has been incorporated, with increased diagnostic weighting given to rapid eye movement (REM) sleep behavior disorder (RBD) and iodine-123-metaiodobenzylguanidine (MIBG) myocardial scintigraphy. Future directions include the development of the criteria for early diagnosis (prodromal DLB) and the establishment of new biomarkers that directly indicate Lewy-related pathology, including α-synuclein imaging, biopsies of peripheral tissues (skin, etc.) for the demonstration of α-synuclein deposition, and biochemical markers (cerebrospinal fluid/blood), as well as the pathological evaluation of the sensitivity and specificity of the 2017 revised diagnostic criteria. In conclusion, the revised consensus criteria for the clinical diagnosis of DLB were reported with the incorporation of new information about DLB in 2017. Future directions include the development of the criteria for early diagnosis and the establishment of biomarkers directly indicative of Lewy-related pathology.
-
Keywords: α-synuclein; Clinical practice guideline; Dementia; Lewy body; Myocardial scintigraphy
INTRODUCTION
- Neurodegenerative diseases characterized by the accumulation of aggregated α-synuclein have been referred to as α-synucleinopathies. Among α-synucleinopathies, Lewy body diseases are characterized by the accumulation of aggregated α-synuclein into Lewy bodies and Lewy neurites in neurons and neuronal processes (Lewy-related pathology) [1]. Lewy body diseases include Parkinson’s disease (PD), PD with dementia (PDD), dementia with Lewy bodies (DLB), and other disorders. Among neurodegenerative diseases causing dementia, DLB is second to Alzheimer’s disease (AD).
- In Lewy body diseases, Lewy-related pathology is distributed in the central and peripheral nervous systems frequently accompanying AD-type neuropathology, which results in the dysfunction and death of synapses and neurons in various areas of the nervous system. Furthermore, patterns of the extension and speed of Lewy-related pathologies are not uniform, including ascending, descending, and olfactory routes that have been considered to be related to the intraneuronal and transsynaptic propagation of α-synuclein aggregates in the nervous system with a prion-like mechanism [2-4]. The complexity of the pathophysiology of Lewy body diseases is linked to considerable variations in clinical manifestations and courses, showing multiple phenotypes, such as PD, PDD, and DLB. DLB is a clinically defined term for dementia with other clinical manifestations characteristic of Lewy-related pathologies, which have been based on a retrospective review of the clinical histories of pathologically confirmed cases.
- Pathological studies with autopsy samples from the general population showed that Lewy body pathology, found in 22.5% of the general population and 41.4% of the demented subjects, often coexists with AD pathology, and aging and AD have strong effects on the evolution of DLB pathology, influencing clinical severity and prognosis [5]. The true prevalence of DLB and the mixed disease of AD with DLB is likely to be much higher than that of clinically recognized cases; DLB diagnoses are often missed [6]. These findings indicate the limitation of current diagnostic criteria and biomarkers to sensitively detect patients with DLB or the mixed dementia of DLB and AD.
- It is required that the criteria for the clinical diagnosis of DLB can detect various clinical phenotypes and laboratory findings of DLB with enough sensitivity and exclude AD and other diseases with high specificity. This article describes a historical review of the criteria for the clinical diagnosis of DLB by the International DLB Consortium, efforts by the authors to upgrade the diagnostic value of 123I-meta-iodobenzylguanidine (MIBG) myocardial scintigraphy, the 2017 revised diagnostic criteria, and future directions to further improve the diagnostic criteria for DLB.
BRIEF HISTORICAL REVIEW OF THE DIAGNOSTIC CRITERIA FOR DLB: THE 1996 ORIGINAL CRITERIA AND 2005 REVISED CRITERIA
- After the first international workshop on DLB held in Newcastle upon Tyne in 1995, the first consensus guidelines were published in 1996, including the consensus criteria for the clinical diagnosis of probable and possible DLB [7]. The 1996 criteria were simple (Table 1). The progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational function was the central feature, always required. There were three core features: fluctuating cognition, visual hallucinations, and parkinsonism; two of the three core features were essential for probable DLB, and one was essential for possible DLB. In addition, supportive features and less likely cases of DLB diagnosis were described as shown in Table 1. After the second international workshop on DLB held in Amsterdam in 1998, the second consensus report was published in 1999 [8]; there was no revision of the diagnostic criteria.
- The third DLB/PDD International Workshop was held in Newcastle upon Tyne in 2003. After this meeting, the third report of the DLB consortium was published in 2005, including the revised criteria for the clinical diagnosis of DLB [9]. In the 2005 revised criteria (Table 2), the central feature and three core features were the same as in the 1996 original criteria. In this revision, “suggestive features” were added, including 1) rapid eye movement (REM) sleep behavior disorder (RBD), 2) severe neuroleptic sensitivity, and 3) low dopamine transporter (DAT) uptake in basal ganglia demonstrated by single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging, such as 123iodine-labeled N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl) nortropane (123I-FP-CIT) SPECT. If one or more suggestive features were present in the presence of one core feature, a diagnosis of probable DLB could be made. It should be noted that, in addition to clinical features, biomarkers were included in this revised criteria as suggestive and supportive features.
- In 2006, the fourth International Workshop on DLB and PDD was held in Yokohama. As it was just after the publication of the 2005 revised criteria, there was no revision of the criteria.
- In December 2015, the International DLB Conference was held in Fort Lauderdale, Florida. Based on the discussion at the Florida conference incorporated with new information on DLB, the fourth consensus report of the DLB Consortium in 2017 was published, including the new diagnostic criteria [10].
STUDIES FOR UPGRADING 123I-MIBG MYOCARDIAL SCINTIGRAPHY IN THE DIAGNOSTIC CRITERIA FOR DLB
- The usefulness of 123I-MIBG myocardial scintigraphy for the clinical diagnosis of DLB has been reported since 2001 by our group and other groups as single-center studies [11-14]. 123I-MIBG is an analog of norepinephrine and accumulates in sympathetic nerve terminals soon after intravenous administration. The MIBG uptake reflects the density, distribution, and activity of postganglionic sympathetic nerves that are involved in Lewy body diseases, including PD and DLB. As a measure of cardiac MIBG uptake, the heart to mediastinum (H/M) ratio is calculated in early (20 minutes) and delayed (3 hours) images after the intravenous injection of 123I-MIBG (Figure 1A). As patients with DLB, but not AD, frequently present with postganglionic sympathetic nerve lesions caused by Lewy-related pathology, the myocardial uptake of MIBG is clearly and diffusely reduced in DLB compared with AD in early and delayed images, showing a significant difference in the H/M ratio between AD and DLB (Figure 1B). A meta-analysis of single-center studies showed very high sensitivity, 98%, and very high specificity, 94% [15]. However, it should be noted that, except for Lewy body diseases, cardiac diseases (heart failure, ischemic heart disease, etc.), metabolic or endocrine diseases (diabetes mellitus, pheochromocytoma, etc.), neurological diseases with postganglionic sympathetic nerve lesions (peripheral neuropathies such as diabetic neuropathy and amyloid neuropathy), and the use of some medicines (sympathomimetics, tricyclic antidepressants, labetalol, reserpine, etc.) are associated with a reduction in the cardiac uptake of MIBG and may give false-positive MIBG results [14].
- In the 2005 revised criteria, MIBG myocardial scintigraphy was classified as one of the supportive features and not as a suggestive feature such as DAT imaging. To increase the diagnostic weighting of MIBG in the criteria, 1) the standardization of the MIBG techniques, 2) a multicenter study with standardized MIBG, and 3) the direct comparison of MIBG data with pathological findings were required.
- First, we standardized data acquisition and processing methods for 123I-MIBG imaging. Differences among collimators in institutions were standardized by using a calibration phantom [16,17]. The setting of regions of interests (ROIs) for the heart and mediastinum was semiautomated using “smartMIBG” (standardized method for automatic ROI setting in MIBG study) [18]. These methods made it possible to obtain standardized data of the H/M ratio from different institutions. At present, the calibration phantom method has further been extended to European countries to overcome differences in H/M ratios depending on camera-collimator combinations [19].
- Next, we performed a prospective multicenter study to evaluate cardiac sympathetic function for the diagnosis of DLB, involving 10 institutions in Japan. Independent committees performed clinical and MIBG assessments. A total of 133 patients were registered, including probable DLB, possible DLB, and probable AD cases. The DLB diagnoses were based on the 2005 revised criteria [9]. The first baseline study was based on the baseline MIBG data and baseline diagnoses [20]. The second three-year follow-up study was based on baseline MIBG data and the final clinical diagnosis 3 years after [21]. ROC curves for the detection of probable DLB from probable AD with early and delayed H/M ratios at baseline MIBG on the basis of baseline and 3-year follow-up diagnoses are shown in Figure 2. The three-year follow-up improved the ROC curve, giving a sensitivity of 77% and a specificity of 97%. Among patients with the diagnosis of possible DLB at baseline (n = 10), the diagnosis was changed to probable DLB (n = 6), probable AD (n = 1), and other disease (depression) (n = 1), while the other two remained as possible DLB during the 3-year follow-up. Five of six patients who were diagnosed with possible DLB at baseline and with probable DLB at follow-up had a reduced H/M ratio at baseline. Two patients who were diagnosed with possible DLB at baseline and with other diagnoses (AD or depression) at follow-up showed no significant reduction in H/M ratio at baseline. Two patients who were diagnosed with possible DLB at baseline and remained with possible DLB at follow-up showed a reduced H/M ratio at baseline. Thus, a reduction in MIBG uptake may be useful for diagnosis in the early stage of DLB [21].
- Pathologically, Lewy body diseases are associated with the deposition of phosphorylated α-synuclein in cardiac sympathetic nerves and sympathetic ganglia and a marked loss of tyrosine hydroxylase (TH)-positive sympathetic nerve fibers in the heart walls [22]. It was revealed that cardiac MIBG uptake for early and delayed images was correlated with the proportion of residual cardiac sympathetic TH-positive nerve fibers at autopsy [23]. Thus, it was established that a reduction in cardiac MIBG uptake is a marker of postganglionic sympathetic nerve lesions caused by Lewy-related pathology. Based on the high diagnostic specificity in our multicenter study with standardized techniques and pathological evidence, the weighting of MIBG was upgraded in the revised 2017 criteria for the clinical diagnosis of DLB.
THE 2017 REVISED CRITERIA FOR THE CLINICAL DIAGNOSIS OF DLB
- The points of revision in the 2017 criteria [10] are as follows: 1) the 2017 criteria distinguish clearly between clinical features and diagnostic biomarkers, and 2) significant new information about previously reported aspects of DLB has been incorporated into the 2017 revised criteria, with increased diagnostic weighting given to RBD and MIBG.
- The 2017 criteria are shown in Table 3 [10]. The central feature is dementia. The other features are divided into clinical features and biomarkers. Clinical features are divided into core and supportive features. Biomarkers are divided into indicative and supportive biomarkers.
- For dementia as the central feature, prominent memory impairment may not occur in the early stages of DLB, but deficits of attention, executive function, and visuoperceptual ability may be prominent.
- As a core clinical feature, RBD is added to the three core features (fluctuating cognition, visual hallucinations, and parkinsonism) of the previous 2005 criteria. Fluctuation is typically delirium-like, occurring as spontaneous alterations in cognition, attention, and arousal. It is recommended to document at least one measure of fluctuation when applying DLB diagnostic criteria [24-27]. Visual hallucinations are typically well formed, featuring people, children or animals, sometimes accompanied by related phenomena such as passage hallucinations, a sense of presence, and visual illusions. For parkinsonism, parkinsonism in DLB frequently falls short of that in PD characterized by combinations of bradykinesia, resting tremor, and rigidity; one or more spontaneous features of parkinsonism are required in the DLB diagnostic criteria. RBD is characterized by recurrent dream enactment behavior that includes movements mimicking dream content and is associated with an absence of normal REM sleep atonia. The inclusion of RBD as a core clinical feature has been reported to improve the diagnostic accuracy of DLB based on a study with autopsy-confirmed DLB and non-DLB cases [28], which was sufficient to justify upgrading RBD from a suggestive feature (2005) [9] to a core clinical feature (2017) [10]. RBD may begin many years before and may become quiescent over time. RBD should be screened using a scale that allows for patient or bed partner report [29,30].
- Supportive clinical features may indicate the diagnosis of DLB, although they lack diagnostic specificity. They include severe antipsychotic sensitivity; postural instability; repeated falls; hypersomnia, usually presenting as excessive daytime sleepiness; syncope; transient episodes of unresponsiveness, which may be an extreme form of cognitive fluctuation and difficult to distinguish from true syncope; severe autonomic dysfunction (orthostatic hypotension/constipation/urinary incontinence); hyposmia, which occurs earlier in DLB that in AD; hallucinations in other modalities; systematized delusions; apathy; anxiety; and depression. “Severe sensitivity to antipsychotic agents” was changed from one of the suggestive features in the 2005 criteria [9] to one of the supportive clinical features in the 2017 criteria [10] because a significant reduction in prescribing D2 receptor blocking antipsychotics in DLB limits its diagnostic usefulness, although caution about their use remains unchanged [10].
- Indicative biomarkers are 1) reduced DAT uptake in basal ganglia on PET or SPECT, such as FP-CIT; 2) low MIBG uptake; and 3) the polysomnographic confirmation of RBD, i.e., REM sleep without atonia (RWA). High specificities of reduced uptake in DAT imaging, low MIBG uptake in MIBG myocardial scintigraphy, and RWA on polysomnography (PSG) for DLB diagnosis have been well established [20,21,31,32]. Studies with a direct comparison of 123I-FP-CIT SPECT and 123I-MIBG scintigraphy in the differential diagnosis of DLB and AD or other dementias showed similar diagnostic sensitivity (90% for both methods) and specificity (91% for both methods) [33], higher sensitivity in 123I-FP-CIT SPECT [88.2% (FP-CIT) > 72.6% (MIBG)] and higher specificity in 123I-MIBG scintigraphy [94.4% (MIBG) > 88.9% (FP-CIT)] [34], similar sensitivity in both methods [96% (FP-CIT), 93% (MIBG)] and higher specificity in 123I-MIBG scintigraphy [100% (MIBG) > 76% (FP-CIT)] [35]. 123I-MIBG scintigraphy may be more specific than 123I-FP-CIT SPECT, especially when parkinsonism is the only “core feature exhibited by the patient.” [35] For PSG, RWA on PSG in a person with dementia and a history of RBD is associated with a ≥ 90% likelihood of pathologically proven synucleinopathy [32], indicating a high diagnostic specificity of PSG-confirmed RBD even in the absence of any other core feature or indicative biomarker. In earlier stages, PSG-confirmed idiopathic RBD and even incidental RWA on PSG without RBD may be associated with an early transition to DLB or PD, especially when combined with abnormalities in 123I-FP-CIT SPECT or 123I-MIBG scintigraphy [36-39]; abnormalities in 123I-FP-CIT SPECT, 123I-MIBG scintigraphy, and PSG would be useful to identify prodromal DLB/PD.
- For the diagnosis of probable DLB, “two or more core clinical features” or “one core clinical feature, but with one or more indicate biomarkers” is required. For the diagnosis of possible DLB, “one core clinical feature” or “one or more indicative biomarkers, but no core clinical features” is necessary. The diagnosis of probable DLB is not allowed on the basis of only biomarkers.
- Regarding the terms DLB and PDD, in a practice setting, DLB is used when dementia occurs before or concurrently with parkinsonism, and PDD is used when dementia occurs in the context of well-established PD. In a research setting, to distinguish DLB from PDD, the 1-year rule between the onset of dementia and parkinsonism is recommended; DLB is used when dementia occurs before or within one year after the onset of parkinsonism; the 1-year rule is operational with no scientific basis; and DLB and PDD have been considered to represent phenotypic differences in a spectrum of Lewy body diseases.
- To improve the diagnosis of Lewy body dementias (DLB or PDD), assessment toolkits were developed to be easy to use by clinicians and to align with the consensus diagnostic criteria for DLB [40] and were updated for the 2017 revised criteria [41].
FUTURE DIRECTIONS TO IMPROVE THE DIAGNOSTIC CRITERIA FOR DLB
- To further improve the 2017 revised diagnostic criteria for DLB, future directions include 1) the evaluation of the sensitivity and specificity of the 2017 revised criteria by pathologically confirmed cases; 2) the development of the criteria for prodromal DLB, and, further, preclinical DLB to detect early-stage disease; and 3) the establishment of new biomarkers as well as further characterization of clinical features specific to DLB.
- Concerning the pathological evaluation of the criteria for clinical diagnosis, mixed pathologies, especially the frequent co-occurrence of AD-related pathology, complicate the pathophysiological and clinical manifestations of DLB [42,43]. In the diagnostic criteria, the likelihood that the pathological findings are associated with a typical DLB clinical syndrome is assessed on the basis of the staging of Lewy-related pathology and AD pathology [10]. It is recommended to measure reliable biomarkers for AD pathology for the precise clinical diagnosis of DLB. In addition, the distribution of the Lewy-related pathology responsible for each cognitive/psychiatric symptom should be further elucidated with careful clinicopathological studies [44].
- The diagnostic criteria for prodromal DLB are under development [45-48]. The diversity in the clinical course of DLB is related to the difficulty in developing the diagnostic criteria for prodromal DLB. Clinical manifestations at the prodromal stage include mild cognitive impairment (MCI) (particularly, non-amnestic MCI characterized by attentional, executive, and visuo-constructive dysfunctions), RBD, behavioral and psychiatric symptoms (hallucinations, depression, etc.), delirium, mild parkinsonian signs, autonomic dysfunction (constipation, orthostatic hypotension, etc.), and hyposmia. It was reported for MCI with Lewy bodies (MCI-LB) that the presence of supportive neuropsychiatric clinical features identified in the 2017 DLB diagnostic criteria was helpful in differentiating MCI-LB from MCI-AD [49,50]. Neuroimaging and biochemical markers that are currently available [48,51] or under development (as described below) would offer great potential for early diagnosis at the prodromal and, further, preclinical stages of DLB and require further studies. Prospective longitudinal studies with pathological verification are essential to establish the diagnostic criteria for prodromal DLB. Methods to develop consensus criteria for prodromal DLB were discussed in the International Lewy Body Dementia Conference held in Las Vegas, Nevada, in June 2019.
- No disease-modifying therapies (DMTs) are available for α-synucleinopathies as yet, although the development of DMTs targeting α-synuclein is ongoing with several approaches, including decreasing α-synuclein production with RNA interference, inhibiting α-synuclein aggregation, promoting the intracellular degradation of α-synuclein aggregates via enhancing autophagy or enhancing lysosomal degradation, and promoting the extracellular degradation of α-synuclein via active and passive immunization [52]. At the same time, biomarkers that directly indicate the presence of Lewy-related pathology need to be established. These biomarkers include α-synuclein imaging; the detection of α-synuclein deposition by biopsies of skin and other tissue; cerebrospinal fluid (CSF) and blood biochemical markers such as α-synuclein, phosphorylated α-synuclein, misfolded α-synuclein, and α-synuclein aggregates; and genetic markers such as the α-synuclein gene (SNCA). Concerning the molecular imaging of DLB, there are limitations of current imaging biomarkers, requiring tracers with high affinity for α-synuclein [53].
- The abnormal deposition of α-synuclein can be pathologically revealed in peripheral tissues (Figure 3). For the pathological demonstration of abnormal α-synuclein using biopsies, phosphorylated α-synuclein deposits in skin nerve fibers on skin biopsy have been reported to be a sensitive biomarker for DLB diagnosis. It was reported that phosphorylated α-synuclein deposits were detected in the skin nerve fibers of all patients with DLB, but no deposits were detected in healthy controls or in patients with non-synucleinopathy dementia [54]. Among the synucleinopathies, DLB showed the highest phosphorylated α-synuclein load with a widespread involvement of autonomic skin fibers compared with PD or multiple system atrophy [55]. Furthermore, phosphorylated α-synuclein deposits could be detected with skin biopsies in 75% of idiopathic RBD patients, suggesting the possibility of usefulness even in the prodromal stage of DLB, such as idiopathic (isolated) RBD [56]. We need a standardization of the techniques used for α-synuclein immunohistochemistry and multicenter studies to establish the diagnostic value of biopsies of skin or other peripheral tissues.
- For the biochemical detection of α-synuclein in biofluids, decreased CSF levels of total α-synuclein and increased CSF levels of oligomeric α-synuclein in DLB have been reported, however, there are tremendous overlaps between levels in DLB patients and in controls; the combination of α-synuclein species and AD biomarkers (Aβ1-42/tau) improves the diagnostic performance to differentiate DLB from AD [57-59]. For plasma α-synuclein as a blood biomarker, analytical limitations still remain. Assays for the sensitive detection of pathological species of α-synuclein, misfolded or aggregated α-synuclein are under development. It was reported that real-time quaking-induced conversion (RT-QuIC) and protein misfolding cyclic amplification (PMCA) methods allow the amplification of α-synuclein misfolded aggregates in CSF with high sensitivity and specificity [60-62]. It was shown with a PMCA assay of CSF that the sensitivity for the detection of DLB was 100% and that the specificity was more than 90% [62]. We need confirmation of the usefulness of these CSF assays by multicenter studies with standardized RT-QuIC/PMCA methods. Furthermore, CSF exosomes from patients with PD and DLB were reported to contain a pathogenic species of α-synuclein, which could initiate the oligomerization of soluble α-synuclein in target cells, suggesting the possibility of a pathogenic biomarker [63]. In addition, a recent study with gastrointestinal biopsies reported good agreement between α-synuclein immunohistochemistry and a PMCA of α-synuclein aggregates using biopsy samples [64].
CONCLUSIONS
- The revised consensus criteria for the clinical diagnosis of DLB were published in 2017 with the incorporation of new information about DLB. For earlier and more likely diagnoses of DLB, it is required to elucidate phenotypic variations in Lewy body diseases, including DLB, and to establish reliable diagnostic biomarkers that directly indicate the presence of abnormal α-synuclein accumulations related to Lewy-related pathology.
Notes
-
Conflicts of Interest
Masahito Yamada received honoraria for sponsored lectures and research grants from Fujifilm RI Pharma Co., Ltd., Eisai Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd. Kenichi Nakajima has collaborative research works with Fujifilm RI Pharma Co. Ltd. and received research funds for joint research and honoraria for lectures and writing. Mitsuhiro Yoshita received honoraria for sponsored lectures from Fujifilm RI Pharma Co., Ltd. and Nihon Medi-Physics Co., Ltd. Junji Komatsu, Miharu Samuraki-Yokohama, Keiko Nakamura, and Kenji Sakai declare that they have no conflicts of interest.
-
Author Contributions
Conceptualization and data curation: All authors. Writing—original draft: Masahito Yamada. Writing—review & editing: Masahito Yamada, Junji Komatsu, Keiko Nakamura, Kenji Sakai, Kenichi Nakajima, and Mitsuhiro Yoshita.
Acknowledgments
- This work was supported by a grant from the Japan Foundation for Neuroscience and Mental Health. The authors thank all the members of the Prospective Multicenter Study for Evaluation of Cardiac Sympathetic Function for the Diagnosis of DLB in Japan [20, 21] and of the International DLB Consortium for the fourth consensus report [10] for their great contributions. We also thank Ms. Etsuko Tsujiguchi for her excellent secretarial work.
Figure 1.Iodine-123–metaiodobenzylguanidine (123I-MIBG) myocardial scintigraphy and dementia with Lewy bodies (DLB). A: Regions of interest are set on heart (H) (circle in A) and mediastinum (M) (square in A), and myocardial uptake of MIBG is measured as the “heart to mediastinum (H/M) ratio” in early and delayed images. B: Myocardial uptake of MIBG is significantly reduced in DLB (B-3 and B-4) compared with Alzheimer’s disease (AD) (B-1 and B-2) in early (B-1 and B-3) and delayed images (B-2 and B-4).
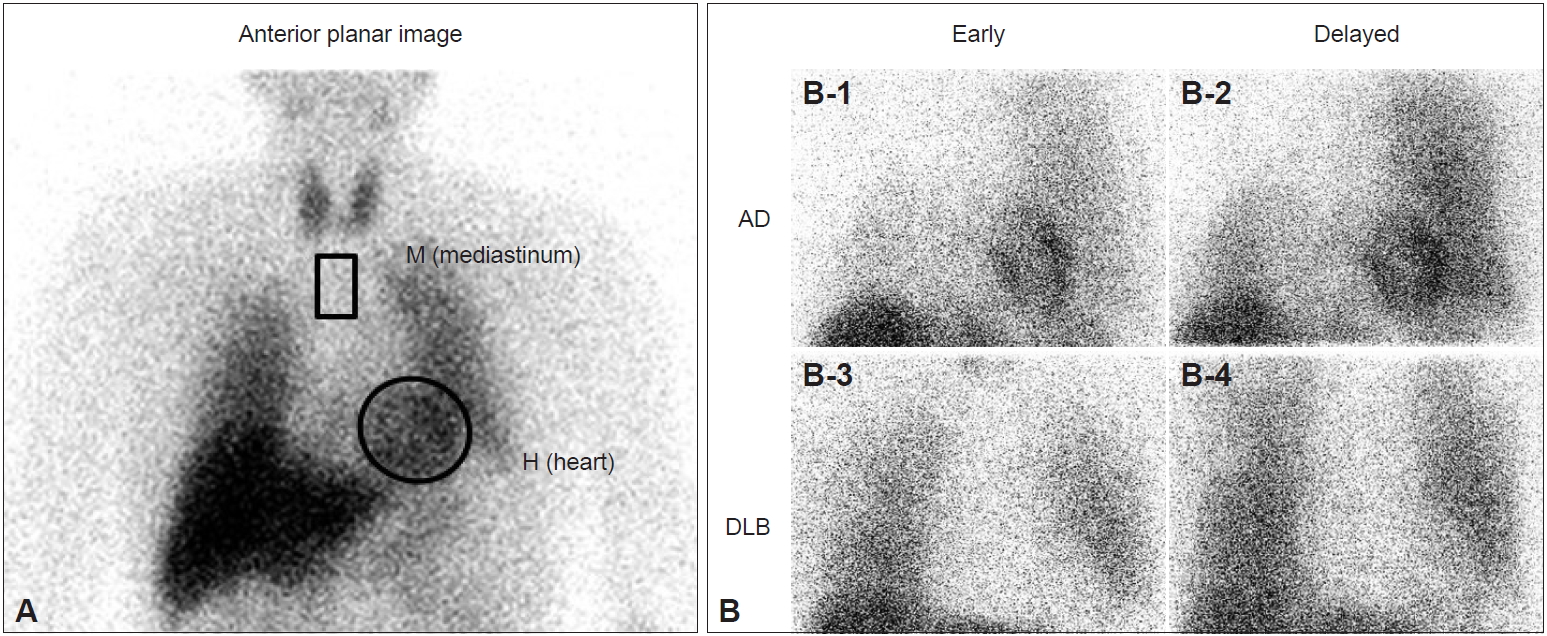
Figure 2.Receiver operating characteristic (ROC) curves for the differentiation of probable dementia with Lewy bodies (DLB) from probable Alzheimer’s disease (AD) based on the early (A) and delayed (B) heart to mediastinum (H/M) ratio of iodine-123 –metaiodobenzylguanidine (123I-MIBG) cardiac scintigraphy at baseline. ROC curves with 3-year follow-up diagnoses are shown by black lines in both the early and delayed images (A and B), and those with baseline diagnoses are shown by red line for the early image (A) and gray line for the delayed image (B). ROC curves with 3-year follow-up diagnoses are superior to those with baseline diagnoses in both the early and delayed images. The ROC curves with 3-year follow-up diagnosis give an area under the curve (AUC) of 0.90, a sensitivity of 0.77, a specificity of 0.94, a positive predictive value (PPV) of 0.83, and a negative predictive value (NPV) of 0.87 for the early image (A) and an AUC of 0.92, a sensitivity of 0.77, a specificity of 0.97, a PPV of 0.96, and an NPV of 0.81 for the delayed image (B).

Figure 3.Deposits of phosphorylated α-synuclein (arrows) in the nerve of an intercostal muscle from a patient with dementia with Lewy bodies (DLB). Original data from our study to identify phosphorylated α-synuclein deposition in peripheral tissues from autopsied patients with DLB/Parkinson’s disease (PD). Immunohistochemistry with an antibody to phosphorylated α-synuclein; bar = 50 μm.
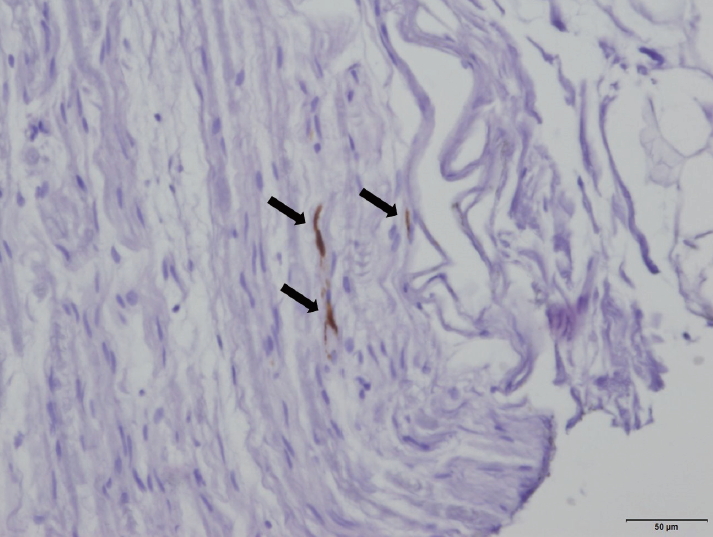
Table 1.Consensus criteria for the clinical diagnosis of probable and possible DLB (1996)
|
1. Central feature: Required
|
|
|
Progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational function |
|
2. Core features: Two are essential for “probable DLB” and one is essential for “possible DLB”
|
|
|
a. Fluctuating cognition with pronounced variations in attention and alertness |
|
|
b. Recurrent visual hallucinations that are typically well formed and detailed |
|
|
c. Spontaneous motor features of parkinsonism |
|
3. Features supportive of the diagnosis are as follows: |
|
|
Repeated falls, syncope, a transient loss of consciousness, neuroleptic sensitivity, systematized delusions, and hallucinations in other modalities |
|
4. A diagnosis of DLB is less likely in the presence of the following: |
|
|
a. Stroke disease, evident as focal neurological signs or on brain imaging |
|
|
b. Evidence on physical examinations and the investigation of any physical illness or other brain disorder sufficient to account for the clinical picture |
Table 2.Revised criteria for the clinical diagnosis of DLB (2005)
|
1. Central feature: Essential for a diagnosis of “possible or probable DLB”
|
|
|
Dementia (deficits on tests of attention, executive function, and visuospatial ability may be especially prominent) |
|
2. Core features: Two are sufficient for “probable DLB” and one is essential for “possible DLB”
|
|
|
a. Fluctuating cognition with pronounced variations in attention and alertness |
|
|
b. Recurrent visual hallucinations that are typically well formed and detailed |
|
|
c. Spontaneous features of parkinsonism |
|
3. Suggestive features: If one or more of these features are present in the presence of one core feature, a diagnosis of “probable DLB” can be made. In the absence of any core features, one or more suggestive features is sufficient for a diagnosis of “possible DLB”. Probable DLB should not be diagnosed on the basis of suggestive features alone.
|
|
|
a. Rapid eye movement (REM) sleep behavior disorder (RBD) |
|
|
b. Severe neuroleptic sensitivity |
|
|
c. Low dopamine transporter uptake in basal ganglia demonstrated by SPECT or PET imaging |
|
4. Supportive features: Commonly present but not proven to have diagnostic specificity
|
|
|
Repeated falls and syncope; a transient, unexplained loss of consciousness; severe autonomic dysfunction (orthostatic hypotension/urinary incontinence); hallucinations in other modalities; systematized delusions; depression; a relative preservation of medial temporal lobe structures on CT/MRI scan; generalized low uptake on SPECT/PET perfusion scan with reduced occipital activity; abnormal (low uptake) MIBG myocardial scintigraphy; and prominent slow-wave activity on EEG with temporal lobe transient sharp waves |
|
5. A diagnosis of DLB is less likely: |
|
|
a. In the presence of cerebrovascular disease evident as focal neurological signs or on brain imaging |
|
|
b. In the presence of any other physical illness or brain disorder sufficient to account in part or in total for the clinical picture |
|
|
c. If parkinsonism only appears for the first time at a stage of severe dementia |
|
6. Temporal sequence of symptoms: |
|
|
“One-year rule” between the onset of dementia and parkinsonism for distinction between DLB and PDD |
Table 3.Revised criteria for the clinical diagnosis of probable and possible dementia with Lewy bodies (DLB) (2017)
|
○ Central feature: Essential for a diagnosis of DLB
|
|
|
Dementia. In the early stages, prominent memory impairment may not occur, but deficits of attention, executive function, and visuoperceptual ability may be prominent. |
|
○ Core clinical features (The first 3 typically occur early and may persist throughout the course) |
|
|
• Fluctuating cognition with pronounced variations in attention and alertness |
|
|
• Recurrent visual hallucinations that are typically well formed and detailed |
|
|
• Rapid eye movement (REM) sleep behavior disorder (RBD), which may precede cognitive decline |
|
|
• One or more spontaneous cardinal features of parkinsonism: bradykinesia, resting tremor, or rigidity |
|
○ Supportive clinical features
|
|
|
Severe sensitivity to antipsychotic agents; postural instability; repeated falls; syncope or other transient episodes of unresponsiveness; severe autonomic dysfunction (e.g., constipation, orthostatic hypotension, or urinary incontinence); hypersomnia; hyposmia; hallucinations in other modalities; systematized delusions; and apathy, anxiety, and depression |
|
○ Indicative biomarkers
|
|
|
• Reduced dopamine transporter uptake in basal ganglia demonstrated by SPECT/PET |
|
|
• Abnormal (low uptake) 123I-MIBG myocardial scintigraphy |
|
|
• Polysomnographic confirmation of REM sleep without atonia |
|
○ Supportive biomarkers
|
|
|
A relative preservation of medial temporal lobe structures on CT/MRI scan; generalized low uptake on SPECT/PET perfusion/metabolism scan with reduced occipital activity ± the cingulate island sign on FDG-PET imaging; prominent posterior slow-wave activity on EEG with periodic fluctuations in the pre-alpha/theta range |
|
Probable DLB
|
|
|
a. Two or more core clinical features are present, or |
|
|
b. Only one core clinical feature is present, but with one or more indicative biomarkers |
|
Possible DLB
|
|
|
a. Only one core clinical feature, or |
|
|
b. One or more indicative biomarkers is present, but there are no core clinical features |
|
DLB is less likely
|
|
|
a. In the presence of any other physical illness or brain disorder, including cerebrovascular disease, sufficient to account in part or in total for the clinical picture, although these do not exclude a DLB diagnosis and may serve to indicate mixed or multiple pathologies contributing to the clinical presentation, or |
|
|
b. If parkinsonian features are the only core clinical feature and appear for the first time at a stage of severe dementia. |
|
DLB and PDD
|
|
|
DLB should be diagnosed when dementia occurs before or concurrently with parkinsonism. The term PDD should be used to describe dementia that occurs in the context of well-established Parkinson’s disease. In a practice setting, the term that is most appropriate to the clinical situation should be used, and generic terms such as Lewy body disease are often helpful. In research studies in which distinction needs to be made between DLB and PDD, the existing 1-year rule between the onset of dementia and parkinsonism continues to be recommended. |
REFERENCES
- 1. Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, et al. Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 2019;14:5.ArticlePubMedPMCPDF
- 2. Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, et al. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol Commun 2014;2:88.ArticlePubMedPMCPDF
- 3. Uchihara T, Giasson BI. Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 2016;131:49–73.ArticlePubMedPDF
- 4. Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, et al. Transneuronal propagation of pathologic α-Synuclein from the gut to the brain models Parkinson’s disease. Neuron 2019;103:627–641.e7. ArticlePubMedPMC
- 5. Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T. Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol 2003;106:374–382.ArticlePubMedPDF
- 6. Vann Jones SA, O’Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med 2014;44:673–683.ArticlePubMed
- 7. McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113–1124.ArticlePubMed
- 8. McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology 1999;53:902–905.ArticlePubMed
- 9. McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 2005;65:1863–1872.ArticlePubMed
- 10. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 2017;89:88–100.ArticlePubMedPMC
- 11. Yoshita M, Taki J, Yamada M. A clinical role for [(123)I]MIBG myocardial scintigraphy in the distinction between dementia of the Alzheimer’s-type and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2001;71:583–588.ArticlePubMedPMC
- 12. Watanabe H, Ieda T, Katayama T, Takeda A, Aiba I, Doyu M, et al. Cardiac (123)I-meta-iodobenzylguanidine (MIBG) uptake in dementia with Lewy bodies: comparison with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2001;70:781–783.ArticlePubMedPMC
- 13. Yoshita M, Taki J, Yokoyama K, Noguchi-Shinohara M, Matsumoto Y, Nakajima K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology 2006;66:1850–1854.ArticlePubMed
- 14. Yamada M, Yoshita M, Samuraki M, Komatsu J, Nakajima K. (123)I-Metaiodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies. In: Kosaka K, editor. Dementia with Lewy Bodies clinical and biological aspects. 1st ed. Heidelberg: Springer; 2017:157–170.
- 15. Treglia G, Cason E. Diagnostic performance of myocardial innervation imaging using MIBG scintigraphy in differential diagnosis between dementia with Lewy bodies and other dementias: a systematic review and a meta-analysis. J Neuroimaging 2012;22:111–117.ArticlePubMed
- 16. Nakajima K, Okuda K, Matsuo S, Yoshita M, Taki J, Yamada M, et al. Standardization of metaiodobenzylguanidine heart to mediastinum ratio using a calibration phantom: effects of correction on normal databases and a multicentre study. Eur J Nucl Med Mol Imaging 2012;39:113–119.ArticlePubMedPDF
- 17. Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol 2014;21:970–978.ArticlePubMedPMCPDF
- 18. Okuda K, Nakajima K, Hosoya T, Ishikawa T, Konishi T, Matsubara K, et al. Semi-automated algorithm for calculating heart-to-mediastinum ratio in cardiac Iodine-123 MIBG imaging. J Nucl Cardiol 2011;18:82–89.ArticlePubMedPDF
- 19. Verschure DO, Poel E, Nakajima K, Okuda K, van Eck-Smit BLF, Somsen GA, et al. A European myocardial 123I-mIBG cross-calibration phantom study. J Nucl Cardiol 2018;25:1191–1197.ArticlePubMedPDF
- 20. Yoshita M, Arai H, Arai H, Arai T, Asada T, Fujishiro H, et al. Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: a multicenter study. PLoS One 2015;10:e0120540.ArticlePubMedPMC
- 21. Komatsu J, Samuraki M, Nakajima K, Arai H, Arai H, Arai T, et al. (123) I-MIBG myocardial scintigraphy for the diagnosis of DLB: a multicentre 3-year follow-up study. J Neurol Neurosurg Psychiatry 2018;89:1167–1173.ArticlePubMed
- 22. Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 2008;131(Pt 3):642–650.ArticlePubMedPDF
- 23. Takahashi M, Ikemura M, Oka T, Uchihara T, Wakabayashi K, Kakita A, et al. Quantitative correlation between cardiac MIBG uptake and remaining axons in the cardiac sympathetic nerve in Lewy body disease. J Neurol Neurosurg Psychiatry 2015;86:939–944.ArticlePubMed
- 24. Bradshaw J, Saling M, Hopwood M, Anderson V, Brodtmann A. Fluctuating cognition in dementia with Lewy bodies and Alzheimer’s disease is qualitatively distinct. J Neurol Neurosurg Psychiatry 2004;75:382–387.ArticlePubMedPMC
- 25. Ferman TJ, Smith GE, Boeve BF, Ivnik RJ, Petersen RC, Knopman D, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004;62:181–187.ArticlePubMed
- 26. Walker MP, Ayre GA, Cummings JL, Wesnes K, McKeith IG, O’Brien JT, et al. The clinician assessment of fluctuation and the one day fluctuation assessment scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry 2000;177:252–256.ArticlePubMed
- 27. Lee DR, McKeith I, Mosimann U, Ghosh-Nodial A, Grayson L, Wilson B, et al. The dementia cognitive fluctuation scale, a new psychometric test for clinicians to identify cognitive fluctuations in people with dementia. Am J Geriatr Psychiatry 2014;22:926–935.ArticlePubMed
- 28. Ferman TJ, Boeve BF, Smith GE, Lin SC, Silber MH, Pedraza O, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology 2011;77:875–882.ArticlePubMedPMC
- 29. Boeve BF, Molano JR, Ferman TJ, Smith GE, Lin SC, Bieniek K, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med 2011;12:445–453.ArticlePubMedPMC
- 30. Postuma RB, Arnulf I, Hogl B, Iranzo A, Miyamoto T, Dauvilliers Y, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord 2012;27:913–916.ArticlePubMedPMC
- 31. McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007;6:305–313.ArticlePubMed
- 32. Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 2013;14:754–762.ArticlePubMedPMC
- 33. Treglia G, Cason E, Cortelli P, Gabellini A, Liguori R, Bagnato A, et al. Iodine-123 metaiodobenzylguanidine scintigraphy and iodine-123 ioflupane single photon emission computed tomography in Lewy body diseases: complementary or alternative techniques? J Neuroimaging 2014;24:149–154.ArticlePubMed
- 34. Shimizu S, Hirao K, Kanetaka H, Namioka N, Hatanaka H, Hirose D, et al. Utility of the combination of DAT SPECT and MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2016;43:184–192.ArticlePubMedPDF
- 35. Tiraboschi P, Corso A, Guerra UP, Nobili F, Piccardo A, Calcagni ML, et al. (123)I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane single photon emission computed tomography and (123)I-metaiodobenzylguanidine myocardial scintigraphy in differentiating dementia with lewy bodies from other dementias: a comparative study. Ann Neurol 2016;80:368–378.ArticlePubMed
- 36. Iranzo A, Santamaría J, Valldeoriola F, Serradell M, Salamero M, Gaig C, et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 2017;82:419–428.ArticlePubMed
- 37. Fujishiro H, Okuda M, Iwamoto K, Miyata S, Torii Y, Iritani S, et al. Early diagnosis of Lewy body disease in patients with late-onset psychiatric disorders using clinical history of rapid eye movement sleep behavior disorder and [(123)I]-metaiodobenzylguanidine cardiac scintigraphy. Psychiatry Clin Neurosci 2018;72:423–434.ArticlePubMed
- 38. Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain 2019;142:2051–2067.ArticlePubMedPDF
- 39. Fujishiro H, Okuda M, Iwamoto K, Miyata S, Torii Y, Iritani S, et al. Clinical profiles of late-onset psychiatric patients exhibiting incidental REM sleep without atonia. J Neural Transm (Vienna) 2019;126:1095–1104.ArticlePubMedPDF
- 40. Thomas AJ, Taylor JP, McKeith I, Bamford C, Burn D, Allan L, et al. Development of assessment toolkits for improving the diagnosis of the Lewy body dementias: feasibility study within the DIAMOND Lewy study. Int J Geriatr Psychiatry 2017;32:1280–1304.ArticlePubMed
- 41. Thomas AJ, Taylor JP, McKeith I, Bamford C, Burn D, Allan L, et al. Revision of assessment toolkits for improving the diagnosis of Lewy body dementia: the DIAMOND Lewy study. Int J Geriatr Psychiatry 2018;33:1293–1304.ArticlePubMedPMC
- 42. Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017;16:55–65.ArticlePubMedPMC
- 43. Ferman TJ, Aoki N, Crook JE, Murray ME, Graff-Radford NR, van Gerpen JA, et al. The limbic and neocortical contribution of α-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimers Dement 2018;14:330–339.ArticlePubMed
- 44. Sakai K, Ikeda T, Ishida C, Komai K, Yamada M. Delusions and visual hallucinations in a patient with Parkinson’s disease with dementia showing pronounced Lewy body pathology in the nucleus basalis of Meynert. Neuropathology 2019;39:319–323.ArticlePubMed
- 45. Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther 2014;6:46.ArticlePubMedPMC
- 46. Fujishiro H, Nakamura S, Sato K, Iseki E. Prodromal dementia with Lewy bodies. Geriatr Gerontol Int 2015;15:817–826.ArticlePubMed
- 47. McKeith I, Taylor JP, Thomas A, Donaghy P, Kane J. Revisiting DLB diagnosis: a consideration of prodromal DLB and of the diagnostic overlap with Alzheimer disease. J Geriatr Psychiatry Neurol 2016;29:249–253.ArticlePubMed
- 48. Durcan R, Donaghy P, Osborne C, Taylor JP, Thomas AJ. Imaging in prodromal dementia with Lewy bodies: where do we stand? Int J Geriatr Psychiatry 2019;34:635–646.ArticlePubMed
- 49. Donaghy PC, Barnett N, Olsen K, Taylor JP, McKeith IG, O’Brien JT, et al. Symptoms associated with Lewy body disease in mild cognitive impairment. Int J Geriatr Psychiatry 2017;32:1163–1171.ArticlePubMed
- 50. Donaghy PC, Taylor JP, O’Brien JT, Barnett N, Olsen K, Colloby SJ, et al. Neuropsychiatric symptoms and cognitive profile in mild cognitive impairment with Lewy bodies. Psychol Med 2018;48:2384–2390.ArticlePubMed
- 51. Thomas AJ, Donaghy P, Roberts G, Colloby SJ, Barnett NA, Petrides G, et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med 2019;49:396–402.ArticlePubMed
- 52. Savitt D, Jankovic J. Targeting α-synuclein in Parkinson’s disease: progress towards the development of disease-modifying therapeutics. Drugs 2019;79:797–810.ArticlePubMedPDF
- 53. Cousins O, Yousaf T, Wilson H, Pagano G, Politis M. Molecular imaging of dementia with Lewy bodies. Int Rev Neurobiol 2019;144:59–93.ArticlePubMed
- 54. Donadio V, Incensi A, Rizzo G, Capellari S, Pantieri R, Stanzani Maserati M, et al. A new potential biomarker for dementia with Lewy bodies: Skin nerve α-synuclein deposits. Neurology 2017;89:318–326.ArticlePubMed
- 55. Donadio V, Incensi A, El-Agnaf O, Rizzo G, Vaikath N, Del Sorbo F, et al. Skin α-synuclein deposits differ in clinical variants of synucleinopathy: an in vivo study. Sci Rep 2018;8:14246.ArticlePubMedPMCPDF
- 56. Antelmi E, Donadio V, Incensi A, Plazzi G, Liguori R. Skin nerve phosphorylated α-synuclein deposits in idiopathic REM sleep behavior disorder. Neurology 2017;88:2128–2131.ArticlePubMed
- 57. van Steenoven I, Majbour NK, Vaikath NN, Berendse HW, van der Flier WM, van de Berg WDJ, et al. α-Synuclein species as potential cerebrospinal fluid biomarkers for dementia with lewy bodies. Mov Disord 2018;33:1724–1733.ArticlePubMedPMC
- 58. Parnetti L, Paciotti S, Farotti L, Bellomo G, Sepe FN, Eusebi P. Parkinson’s and Lewy body dementia CSF biomarkers. Clin Chim Acta 2019;495:318–325.ArticlePubMed
- 59. Bousiges O, Blanc F. Diagnostic value of cerebro-spinal fluid biomarkers in dementia with lewy bodies. Clin Chim Acta 2019;490:222–228.ArticlePubMed
- 60. Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 2016;3:812–818.ArticlePubMedPMC
- 61. Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun 2018;6:7.ArticlePubMedPMCPDF
- 62. Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, Trenkwalder C, et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 2017;74:163–172.ArticlePubMed
- 63. Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, et al. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain 2016;139(Pt 2):481–494.ArticlePubMedPDF
- 64. Fenyi A, Leclair-Visonneau L, Clairembault T, Coron E, Neunlist M, Melki R, et al. Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol Dis 2019;129:38–43.ArticlePubMed
Citations
Citations to this article as recorded by

- The Role of Electroconvulsive Therapy in the Treatment of Catatonia Associated With Lewy Body Dementia: A Case Report
Javeria Sahib Din, Thomas Boes, Ernesto Navarro Garcia, Hiba Al-Rubaye
Cureus.2024;[Epub] CrossRef - α-Synuclein pathology from the body to the brain: so many seeds so close to the central soil
Yunying Yang, Zhentao Zhang
Neural Regeneration Research.2024; 19(7): 1463. CrossRef - Suspecting Non-Alzheimer’s Pathologies and Mixed Pathologies: A Comparative Study Between Brain Metabolism and Tau Images
Vincent Malotaux, Lise Colmant, Lisa Quenon, Lara Huyghe, Thomas Gérard, Laurence Dricot, Adrian Ivanoiu, Renaud Lhommel, Bernard Hanseeuw
Journal of Alzheimer's Disease.2024; 97(1): 421. CrossRef - Supercomplex formation of mitochondrial respiratory chain complexes in leukocytes from patients with neurodegenerative diseases
Tsukasa Hara, Ryosuke Amagai, Ryuji Sakakibara, Ayako Okado-Matsumoto
The Journal of Biochemistry.2024; 175(3): 289. CrossRef - Latest advances in mechanisms of epileptic activity in Alzheimer’s disease and dementia with Lewy Bodies
Mariane Vicente, Kwaku Addo-Osafo, Keith Vossel
Frontiers in Neurology.2024;[Epub] CrossRef - The clinical diagnosis of Parkinson's disease
Renato P. Munhoz, Vitor Tumas, José Luiz Pedroso, Laura Silveira-Moriyama
Arquivos de Neuro-Psiquiatria.2024; 82(06): 001. CrossRef - Identification of Potentially Repurposable Drugs for Lewy Body Dementia Using a Network-Based Approach
Megha Manoj, Siddarth Sowmyanarayan, Arjun V. Kowshik, Jhinuk Chatterjee
Journal of Molecular Neuroscience.2024;[Epub] CrossRef - Altered structural and functional connectivity in Posterior Cortical Atrophy and Dementia with Lewy bodies
Neha Atulkumar Singh, Austin W. Goodrich, Jonathan Graff-Radford, Mary M. Machulda, Irene Sintini, Arenn F. Carlos, Carling G. Robinson, Robert I. Reid, Val J. Lowe, Clifford R. Jack, Ronald C. Petersen, Bradley F. Boeve, Keith A. Josephs, Kejal Kantarci,
NeuroImage.2024; 290: 120564. CrossRef - Clinicopathological correlation of cerebrospinal fluid alpha‐synuclein seed amplification assay in a behavioral neurology autopsy cohort
Niyatee Samudra, D. Luke Fischer, Steven Lenio, Argentina Lario Lago, Peter A. Ljubenkov, Julio C. Rojas, William W. Seeley, Salvatore Spina, Adam M. Staffaroni, Jonathan Tablante, Fattin Wekselman, Jennifer Lamoureux, Luis Concha‐Marambio, Lea T. Grinber
Alzheimer's & Dementia.2024;[Epub] CrossRef - New insight of exercise on dementia; combinatory effects of physical and cognitive exercise
Hyo-Jeong Cha, Jun Hong Park, Changwan Hong
Molecular & Cellular Toxicology.2024;[Epub] CrossRef - Magnetic resonance imaging in the diagnosis of progressive supranuclear palsy: A case report and review of literature
Baraka Alphonce, Francisca Komanya, Mbelwa Bitesigilwe, John R. Meda, Azan Nyundo
Clinical Case Reports.2023;[Epub] CrossRef - Parkinson's disease fluid biomarkers for differential diagnosis of atypical parkinsonian syndromes
Jun Yang, Ayotimofe Idowu, Liana Rosenthal, Xiaobo Mao
Clinical and Translational Discovery.2023;[Epub] CrossRef - Neurofilament light chain is increased in the parahippocampal cortex and associates with pathological hallmarks in Parkinson’s disease dementia
Irene Frigerio, Max A. Laansma, Chen-Pei Lin, Emma J. M. Hermans, Maud M. A. Bouwman, John G. J. M. Bol, Yvon Galis-de Graaf, Dagmar H. Hepp, Annemieke J. M. Rozemuller, Frederik Barkhof, Wilma D. J. van de Berg, Laura E. Jonkman
Translational Neurodegeneration.2023;[Epub] CrossRef - Machine learning-based prediction of conversion coefficients for I-123 metaiodobenzylguanidine heart-to-mediastinum ratio
Koichi Okuda, Kenichi Nakajima, Chiemi Kitamura, Michael Ljungberg, Tetsuo Hosoya, Yumiko Kirihara, Mitsumasa Hashimoto
Journal of Nuclear Cardiology.2023; 30(4): 1630. CrossRef - Costs During the Last Five Years of Life for Patients with Clinical and Pathological Confirmed Diagnosis of Lewy Body Dementia and Alzheimer’s Disease
Carolyn W. Zhu, Yian Gu, Anton J. Kociolek, Kayri K. Fernandez, Stephanie Cosentino, Yaakov Stern
Journal of Alzheimer's Disease.2023; 92(2): 457. CrossRef - Early- and late-onset of isolated rapid eye movement sleep behavior disorder: A retrospective cohort study
Li Zhou, Bei Huang, Jing Wang, Steven WH. Chau, Joey WY. Chan, Jihui Zhang, Mandy WM. Yu, Jessie CC. Tsang, Shirley Xin Li, Vincent CT. Mok, Yun Kwok Wing, Yaping Liu
Sleep Medicine.2023;[Epub] CrossRef - Parkinson Disease Dementia Management: an Update of Current Evidence and Future Directions
Oliver Phillips, Debolina Ghosh, Hubert H. Fernandez
Current Treatment Options in Neurology.2023; 25(5): 93. CrossRef - Rapidly progressive dementia with severe insomnia: an unusual case of progressive supranuclear palsy mimicking dementia with Lewy bodies
Rae On Kim, Eun Ji Lee, Seong-Ik Kim, Sung-Hye Park, Kyum-Yil Kwon
Neurological Sciences.2023; 44(8): 2953. CrossRef - Significance of clinical symptoms and red flags in early differential diagnosis of Parkinson’s disease and atypical Parkinsonian syndromes
Nils Schröter, Thilo van Eimeren, Joseph Classen, Johannes Levin, Christoph Redecker, Martin Wolz, Lars Tönges
Journal of Neural Transmission.2023; 130(6): 839. CrossRef - Electroencephalography in young onset dementia
Casey W Brown, Huei-Yang Chen, Peter K Panegyres
BMC Neurology.2023;[Epub] CrossRef - Clinical trials in dementia with Lewy bodies: the evolving concept of co-pathologies, patient selection and biomarkers
Lucy L. Gibson, Carla Abdelnour, Joyce Chong, Clive Ballard, Dag Aarsland
Current Opinion in Neurology.2023; 36(4): 264. CrossRef - Coexistence of Posterior Cortical Atrophy and Parkinson’s Disease
Eun-Byul Ko, Il-Joong Hwang, Jung-Woo Kim, Dar-Eun Jung, Ju-Suk Lee, Sang-Won Yoo, Joong-Seok Kim
Journal of the Korean Neurological Association.2023; 41(3): 216. CrossRef - Toward a new nosology of neurodegenerative diseases
Manuel Menéndez‐González
Alzheimer's & Dementia.2023; 19(8): 3731. CrossRef - Autonomic dysfunction in dementia with Lewy bodies: Focusing on cardiovascular and respiratory dysfunction
Katsuyoshi Mizukami
Psychiatry and Clinical Neurosciences Reports.2023;[Epub] CrossRef - Beyond Strains: Molecular Diversity in Alpha-Synuclein at the Center of Disease Heterogeneity
Marcelina J. Wojewska, Maria Otero-Jimenez, Jose Guijarro-Nuez, Javier Alegre-Abarrategui
International Journal of Molecular Sciences.2023; 24(17): 13199. CrossRef - Case Study 6: The Diagnostic Challenge of a 75-Year-Old Man Who Had, Then Didn’t Have, Then Did Have Alzheimer’s Disease
Sergio A. Ramírez-Salazar, Cassie MacRae, Mel B. Feany, Michael Miller, Hyun-Sik Yang, Mary-Ellen Meadows, Scott M. McGinnis, David Silbersweig, Seth A. Gale, Kirk R. Daffner
The Journal of Neuropsychiatry and Clinical Neurosciences.2023; 35(4): 325. CrossRef - Cardiac 123I-Metaiodobenzylguanidine (MIBG) Scintigraphy in Parkinson’s Disease: A Comprehensive Review
Jamir Pitton Rissardo, Ana Letícia Fornari Caprara
Brain Sciences.2023; 13(10): 1471. CrossRef - A Unique Perspective on Lead Compounds for Dementia with the Lewy
Body
Menaka Subramani, Amuthalakshmi Sivaperuman, Ramalakshmi Natarajan, Keerthana Dhinakaran
Medicinal Chemistry.2023; 19(10): 946. CrossRef - Three-Dimensional Heart Segmentation and Absolute Quantitation of Cardiac 123I-metaiodobenzylguanidine Sympathetic Imaging Using SPECT/CT
Shintaro Saito, Kenichi Nakajima, Takayuki Shibutani, Hiroshi Wakabayashi, Hiroto Yoneyama, Takahiro Konishi, Hiroshi Mori, Aki Takata, Seigo Kinuya
Annals of Nuclear Cardiology.2023; 9(1): 61. CrossRef - Comparison of Taiwanese and European Calibration Factors for Heart-to-Mediastinum Ratio in Multicenter 123I-mIBG Phantom Studies
Koichi Okuda, Kenichi Nakajima, Guang-Uei Hung, Hao-Ting Wu, Derk O. Verschure, Hein J. Verberne, Chiemi Kitamura
Annals of Nuclear Cardiology.2023; 9(1): 54. CrossRef - Clinical biomarkers for Lewy body diseases
Mai M. Abdelmoaty, Eugene Lu, Rana Kadry, Emma G. Foster, Shaurav Bhattarai, R. Lee Mosley, Howard E. Gendelman
Cell & Bioscience.2023;[Epub] CrossRef - Proteomic comparison between non‐purified cerebrospinal fluid and cerebrospinal fluid‐derived extracellular vesicles from patients with Alzheimer's, Parkinson's and Lewy body dementia
Yael Hirschberg, Natalia Valle‐Tamayo, Oriol Dols‐Icardo, Sebastiaan Engelborghs, Bart Buelens, Roosmarijn E. Vandenbroucke, Yannick Vermeiren, Kurt Boonen, Inge Mertens
Journal of Extracellular Vesicles.2023;[Epub] CrossRef - The connection between cerebral amyloid angiopathy and Alzheimer’s disease
Hans Rolf Jäger
European Radiology.2023; 34(4): 2171. CrossRef - Autonomic symptoms are predictive of dementia with Lewy bodies
Wenzheng Hu, Shuai Liu, Fei Wang, Han Zhu, Xiaoshan Du, Lingyun Ma, Jinghuan Gan, Hao Wu, Xiaodan Wang, Yong Ji
Parkinsonism & Related Disorders.2022; 95: 1. CrossRef - Delusion and Delirium in Neurodegenerative Disorders: An Overlooked Relationship?
Daniele Urso, Valentina Gnoni, Marco Filardi, Giancarlo Logroscino
Frontiers in Psychiatry.2022;[Epub] CrossRef - Clinical Trajectories at the End of Life in Autopsy-Confirmed Dementia Patients With Alzheimer Disease and Lewy Bodies Pathologies
Yian Gu, Anton Kociolek, Kayri K. Fernandez, Stephanie A. Cosentino, Carolyn Wei Zhu, Zhezhen Jin, James B. Leverenz, Yaakov B. Stern
Neurology.2022;[Epub] CrossRef - Diagnostic Performance for Differential Diagnosis of Atypical Parkinsonian Syndromes from Parkinson’s Disease Using Quantitative Indices of 18F-FP-CIT PET/CT
Miju Cheon, Seung Min Kim, Sang-Won Ha, Min Ju Kang, Hea-Eun Yang, Jang Yoo
Diagnostics.2022; 12(6): 1402. CrossRef - The promise of amplification assays for accurate early detection of α-synucleinopathies: A review
Regina Kurapova, Leonidas Chouliaras, John T. O'Brien
Experimental Gerontology.2022; 165: 111842. CrossRef - Progressive Olfactory Impairment and Cardiac Sympathetic Denervation in REM Sleep Behavior Disorder
Annette Janzen, David Vadasz, Jan Booij, Markus Luster, Damiano Librizzi, Martin T. Henrich, Lars Timmermann, Mahboubeh Habibi, Elisabeth Sittig, Geert Mayer, Fanni Geibl, Wolfgang Oertel
Journal of Parkinson's Disease.2022; 12(6): 1921. CrossRef - A Systematic Review and Comparison of Neurocognitive Features of Late-Life Attention-Deficit/Hyperactivity Disorder and Dementia With Lewy Bodies
Jennifer L. Prentice, Morgan J. Schaeffer, Alexandra K. Wall, Brandy L. Callahan
Journal of Geriatric Psychiatry and Neurology.2021; 34(5): 466. CrossRef - Dementia with Lewy bodies in first-generation immigrants in a European memory clinic
Kurt Segers, Florence Benoit, Jean-Marie Meyts, Gérald Glibert, Sophie Levy, Murielle Surquin
Acta Neurologica Belgica.2021; 121(1): 219. CrossRef - The development of new method to differentiate between Dementia with Lewy bodies and Alzheimer’s disease by cerebral perfusion SPECT-comparison to CIScore
Gaku Honda, Shigeki Nagamachi, Masanari Nonokuma, Koichi Takano, Yasuo Kuwabara, Kengo Yoshimitsu, Hitoshi Iida, Koji Ogomori, Hiroaki Kawasaki, Yoshio Tsuboi
Japanese Journal of Radiology.2021; 39(2): 198. CrossRef - Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease
Xue-Bing Ding, Xin-Xin Wang, Dan-Hao Xia, Han Liu, Hai-Yan Tian, Yu Fu, Yong-Kang Chen, Chi Qin, Jiu-Qi Wang, Zhi Xiang, Zhong-Xian Zhang, Qin-Chen Cao, Wei Wang, Jia-Yi Li, Erxi Wu, Bei-Sha Tang, Ming-Ming Ma, Jun-Fang Teng, Xue-Jing Wang
Nature Medicine.2021; 27(3): 411. CrossRef - Mechanisms of Neurodegeneration in Various Forms of Parkinsonism—Similarities and Differences
Dariusz Koziorowski, Monika Figura, Łukasz M. Milanowski, Stanisław Szlufik, Piotr Alster, Natalia Madetko, Andrzej Friedman
Cells.2021; 10(3): 656. CrossRef - Advances in computerized MRI‐based biomarkers in Alzheimer’s disease
Raymond Wong, Yishan Luo, Vincent Chung-tong Mok, Lin Shi
Brain Science Advances.2021; 7(1): 26. CrossRef - Convolutional neural network-based automatic heart segmentation and quantitation in 123I-metaiodobenzylguanidine SPECT imaging
Shintaro Saito, Kenichi Nakajima, Lars Edenbrandt, Olof Enqvist, Johannes Ulén, Seigo Kinuya
EJNMMI Research.2021;[Epub] CrossRef - A direct comparison of the 2005 and 2017 criteria for dementia with Lewy bodies
Kurt Segers, Florence Benoit, Jean‐Marie Meyts, Gerald Glibert, Murielle Surquin
Psychogeriatrics.2020; 20(5): 785. CrossRef - Calibrated scintigraphic imaging procedures improve quantitative assessment of the cardiac sympathetic nerve activity
Koichi Okuda, Kenichi Nakajima, Chiemi Kitamura, Yumiko Kirihara, Mitsumasa Hashimoto, Seigo Kinuya
Scientific Reports.2020;[Epub] CrossRef
 , Junji Komatsu1
, Junji Komatsu1 , Keiko Nakamura1
, Keiko Nakamura1 , Kenji Sakai1
, Kenji Sakai1 , Miharu Samuraki-Yokohama1
, Miharu Samuraki-Yokohama1 , Kenichi Nakajima2
, Kenichi Nakajima2 , Mitsuhiro Yoshita1,3
, Mitsuhiro Yoshita1,3


 KMDS
KMDS
 E-submission
E-submission



 PubReader
PubReader ePub Link
ePub Link Cite
Cite



