Articles
- Page Path
- HOME > J Mov Disord > Volume 10(2); 2017 > Article
-
Case Report
Holmes’ Tremor with Shoulder Pain Treated by Deep Brain Stimulation of Unilateral Ventral Intermediate Thalamic Nucleus and Globus Pallidus Internus - Sabri Aydın1, Huseyin Canaz1, Ezgi Tuna Erdogan2, Nazlı Durmaz3, Barıs Topcular4
-
Journal of Movement Disorders 2017;10(2):92-95.
DOI: https://doi.org/10.14802/jmd.16051
Published online: April 18, 2017
1Department of Neurosurgery, Florence Nightingale Hospital, Istanbul Bilim University, Istanbul, Turkey
2Department of Physiology, Istanbul Bilim University, Istanbul, Turkey
3Department of Neurology, Ankara University, Ankara, Turkey
4Department of Neurology, Florence Nightingale Hospital, Istanbul Bilim University, Istanbul, Turkey
- Corresponding author: Huseyin Canaz, MD, Department of Neurosurgery, Florence Nightingale Hospital, Istanbul Bilim University, Abide-i Hurriyet Street No: 163, Istanbul 34381 Turkey / Tel: +90-212-2244950 / Fax: +90-212-2244982 / E-mail: huseyin.canaz@istanbulbilim.edu.tr
• Received: October 11, 2016 • Revised: November 28, 2016 • Accepted: December 7, 2016
Copyright © 2017 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- A 21-year-old male was admitted with severe right arm and hand tremors after a thalamic hemorrhage caused by a traffic accident. He was also suffering from agonizing pain in his right shoulder that manifested after the tremor. Neurologic examination revealed a disabling, severe, and irregular kinetic and postural tremor in the right arm during target-directed movements. There was also an irregular ipsilateral rest tremor and dystonic movements in the distal part of the right arm. The amplitude was moderate at rest and extremely high during kinetic and intentional movements. The patient underwent left globus pallidum internus and ventral intermediate thalamic nucleus deep brain stimulation. The patient improved by more than 80% as rated by the Fahn-Tolosa-Marin Tremor Rating Scale and Visual Analog Scale six months after surgery.
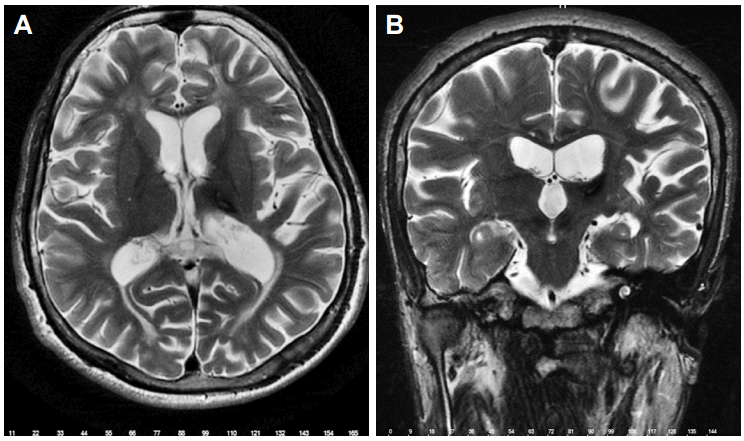
- A 21-year-old male was admitted with severe right arm and hand tremors after a thalamic hemorrhage caused by a traffic accident. He was also suffering from agonizing pain in his right shoulder that manifested after tremor. He had no prior relevant medical history and there were no abnormalities in his general physical findings. No pathology was diagnosed during orthopedic surgery consultation. Neurologic examination revealed disabling, severe, and irregular kinetic and postural tremors in the right arm during target-directed movements. There was also an irregular ipsilateral rest tremor as well as dystonic movements in the distal part of the right arm. The amplitude was moderate at rest and extremely high in kinetic and intentional movements. His baseline severity of tremor was evaluated with Fahn-Tolosa-Marin Tremor Rating Scale (TRS), and the preoperative score was 20 for the proximal and 36 for the distal arm. He marked the intensity of his shoulder pain as 9/10 using the Visual Analog Scale (VAS). Brain MRI showed no abnormality except the thalamic hypointensity in T2-weighted sequences in the left side compatible with the sequalae of the former thalamic hemorrhage (Figure 1). Optimum dosages of levodopa, amantadine, benzodiazepine, and anticholinergic agents did not relieve his tremor.
- The surgical procedure was performed under local anesthesia and sedation using the Leksell (Elekta, Stockholm, Sweden) stereotactic frame with ring system. The surgical targets were the left GPi and Vim. Their positions were localized anatomically by direct visualizing on T2-weighted and inversion recovery MRI. FrameLink 2.0 software (Medtronic, Inc., Minneapolis, MN, USA) was used for the application of the computed tomography (CT)-MR fusion technique, and the MR image was reformatted to the anterior commissure-posterior commissure plane to yield the calculated coordinates for the GPi and Vim with the aid of Schaltenbrand and Wahren stereotactic atlas. Quadripolar electrodes (Model KN 1063, Medtronic, Inc.) were implanted in the left GPi and Vim in a single session. Electrode targeting was confirmed for Vim and GPi using microelectrode recordings (MERs). MERs were performed in three canals (center, medial, and posterior) for Vim and two canals (lateral and center) for GPi. The posterior canal was selected for Vim, and the center canal was selected for GPi according to MERs. Macrostimulation was performed for both targets and demonstrated no side effects. Macroelectrode stimulation showed improvement of more than 60% in the tremors and amelioration of his dystonia.
- The final stereotactic x, y, z, ring, and arc coordinates of the targeted left GPi were 114.1, 108.4, 95.5, 73.7, and 99.5, respectively. For the left Vim they were 116.2, 88.5, 94.2, 52.8, and 111.9, respectively. Following implantation of the DBS lead, he was placed under general anesthesia for implantation of the pulse generator (Medtronic Soletra, Model 7426; Medtronic Neuromodulation). His immediate postoperative TRS was 11 points, and his VAS score was decreased to 3 after the surgery.
- A postoperative cranial CT scan was performed to confirm the position of the electrodes. The electrodes were activated seven days after the surgery. The stimulation parameters were 1 V, 130 Hz, and 90 μs, with monopolar stimulation on contact 0 for both targets. Six months after stimulation, the tremor showed improvement, especially the resting component. The effects of the Vim and GPi stimulation were evaluated separately by individual activation of the leads. Final stimulation adjustments were 3 V, 90 μs, and 100 Hz for the Vim and 3 V, 210 μs, and 130 Hz for the GPi. The final TRS score for his right upper extremity tremor was 3 for the proximal and 4 for the distal arm. His VAS score was 1 at six months after surgery. There were no side effects related to the stimulation.
CASE REPORT
- HT is characterized by a low frequency (typically < 4.5 Hz) tremor with irregular rest, action, and postural components, sometimes with an element of ataxia that presents several weeks to two years after injury. It predominantly affects the upper extremities and, because it includes rest as well as action and postural components, results in severe functional impairment [4]. HT generally does not resolve spontaneously and responds poorly to pharmacological therapy, so surgical therapies have been offered. Ablative therapies such as thalamotomy have not been consistently demonstrated to be beneficial, and the incidence of neurological complications is relatively high [5]. This has led to application of DBS for the treatment of HT.
- The best target for treating HT remains a matter of discussion. In most case reports, the thalamus was selected as a DBS target [6,7].
- All kinds of tremors, including HT, have been shown to be ameliorated by thalamic surgery. Almost 20 HT cases have had Vim DBS with a generally good response but, as with cerebellar tremor, much depends on the extent of the natural lesion and the specific symptom patterns in the individual patient. It has been reported that appendicular tremors respond better than proximal or axial tremors. An exception to the use of Vim as a DBS target for HT, GPi was selected in a single patient for whom Vim DBS was not effective in treating the tremor [3].
- Because GPi has overlapping somatotopy of distal and proximal parts of the extremities, and because it projects both thalamic and brain stem structures related to tremor production, GPi stimulation may be effective in controlling proximal tremor. Our patient was disabled by both distal and proximal tremors. Because Goto and Yamada [8] reported favorable results in a patient with simultaneous pallidotomy and Vim DBS, thus supporting the validity of the approach, and because our previous patient with HT had a satisfactory recovery [1], we decided to implant both Vim and GPi stimulators.
- Even though Vim DBS has been considered a target of choice, different double approaches have been reported in single cases: Vim and STN DBS [9]; ventralis oralis posterior (Vop)/ventralis oralis anterior (Voa) and Vim DBS [6]; Vim DBS and pallidotomy [8], Vim, Voa, and Gpi [3]; and Vim and Gpi DBS [1], as in our previous case, which was based on the hypothesis that HT is caused by the combined imbalance of different cerebral circuits.
- The pathophysiology of HT is complex and full of speculation. It has been shown that impairment of the nigrostriatal pathway is not necessary and damage of cerebello-thalamic pathway is sufficient for HT development [10]. Our patient did not respond to levodopa treatment, proving there were no changes in the nigrostriatal area.
- The outflow pathways from GPi provide a direct influence on not only the thalamus but also the brainstem motor centers, such as the pedunculopontine nucleus, related to the mesencephalic tegmental field that controls the axial and proximal appendicular musculature via the descending reticulospinal tract. Therefore, unlike thalamic surgery, which interrupts the thalamocortical output that controls distal appendicular musculature via descending corticospinal and corticobulbar tracts, GPi pallidal DBS influences the control of otherwise inaccessible axial and proximal muscles. This may be the reason why GPi DBS provides satisfactory improvement of proximal tremors [8].
- A rapid decrease in distal tremors (as expected by thalamic surgery) and a late (within 6 weeks) response of proximal tremors (as expected by GPi surgery both for tremor and dystonia) probably indicate that both targets are effective in our case. This is the third demonstration of GPi + Vim surgery being effective in the treatment of HT. Based on our experience, we propose implanting GPi DBS as well as Vim in patients who have a proximal tremor.
- On the other hand, one of the major complaints made by our patient was pain in his right shoulder, which occurred secondary to his proximal tremor. To the best of our knowledge, this is the first HT patient with shoulder pain in the literature. GPi DBS was also important from this perspective as it aided the satisfactory recovery of his shoulder pain.
- In conclusion, because of the limited efficacy of Vim DBS on proximal tremors, the use of other or additional targets with better effects should be considered for the treatment of HT. To this end, GPi could be considered as an additional target, especially for HT patients with a predominant proximal tremor component.
DISCUSSION
Figure 1.Left thalamic hypointensity due to thalamic hemorrhage in axial T2-weighted sequence on MRI (A) and coronal T2-weighted sequence on MRI (B).


- 1. Aydin S, Abuzayed B, Kiziltan G, Gunduz A, Yagci S, Mengi M, et al. Unilateral thalamic Vim and GPi stimulation for the treatment of Holmes’ tremor caused by midbrain cavernoma: case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg 2013;74:271–276.ArticlePubMedPDF
- 2. Deuschl G, Bergman H. Pathophysiology of nonparkinsonian tremors. Mov Disord 2002;17 Suppl 3:S41–S48.ArticlePubMed
- 3. Lim DA, Khandhar SM, Heath S, Ostrem JL, Ringel N, Starr P. Multiple target deep brain stimulation for multiple sclerosis related and poststroke Holmes’ tremor. Stereotact Funct Neurosurg 2007;85:144–149.ArticlePubMed
- 4. Espinoza Martinez JA, Arango GJ, Fonoff ET, Reithmeier T, Escobar OA, Furlanetti L, et al. Deep brain stimulation of the globus pallidus internus or ventralis intermedius nucleus of thalamus for Holmes tremor. Neurosurg Rev 2015;38:753–763.ArticlePubMed
- 5. Krauss JK, Jankovic J. Head injury and posttraumatic movement disorders. Neurosurgery 2002;50:927–939.discussion 939-940. ArticlePubMed
- 6. Foote KD, Okun MS. Ventralis intermedius plus ventralis oralis anterior and posterior deep brain stimulation for posttraumatic Holmes tremor: two leads may be better than one: technical note. Neurosurgery 2005;56(2 Suppl):E445. discussion E445. ArticlePubMedPDF
- 7. Nikkhah G, Prokop T, Hellwig B, Lücking CH, Ostertag CB. Deep brain stimulation of the nucleus ventralis intermedius for Holmes (rubral) tremor and associated dystonia caused by upper brainstem lesions. Report of two cases. J Neurosurg 2004;100:1079–1083.ArticlePubMed
- 8. Goto S, Yamada K. Combination of thalamic Vim stimulation and GPi pallidotomy synergistically abolishes Holmes’ tremor. J Neurol Neurosurg Psychiatry 2004;75:1203–1204.ArticlePubMedPMC
- 9. Romanelli P, Brontë-Stewart H, Courtney T, Heit G. Possible necessity for deep brain stimulation of both the ventralis intermedius and subthalamic nuclei to resolve Holmes tremor. Case report. J Neurosurg 2003;99:566–571.ArticlePubMed
- 10. Gajos A, Bogucki A, Schinwelski M, Sołtan W, Rudzin´ska M, Budrewicz S, et al. The clinical and neuroimaging studies in Holmes tremor. Acta Neurol Scand 2010;122:360–366.ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Holmes tremor: an updated review
Efstratios-Stylianos Pyrgelis, Eleni Agapiou, Efthalia Angelopoulou
Neurological Sciences.2022; 43(12): 6731. CrossRef - Deep brain stimulation of the posterior subthalamic area as an alternative strategy for management of Holmes tremor: A case report and review of the literature
Omid Yousefi, Mojtaba Dayyani, Razieh Rezaei, Hooman Kamran, Ali Razmkon
Surgical Neurology International.2022; 13: 489. CrossRef - Vim stereotactic radiosurgical thalamotomy for drug-resistant idiopathic Holmes tremor: a case report
Manjul Tripathi, Sahil Mehta, Raghav Singla, Chirag K. Ahuja, Naresh Tandalya, Constantin Tuleasca, Aman Batish, Sandeep Mohindra, Abhinav Agrahari, Rupinder Kaur
Acta Neurochirurgica.2021; 163(7): 1867. CrossRef - Deep brain stimulation in uncommon tremor disorders: indications, targets, and programming
Carlo Alberto Artusi, Ashar Farooqi, Alberto Romagnolo, Luca Marsili, Roberta Balestrino, Leonard L. Sokol, Lily L. Wang, Maurizio Zibetti, Andrew P. Duker, George T. Mandybur, Leonardo Lopiano, Aristide Merola
Journal of Neurology.2018; 265(11): 2473. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite