Articles
- Page Path
- HOME > J Mov Disord > Volume 9(3); 2016 > Article
-
Original Article
Cognition and Visit-to-Visit Variability of Blood Pressure and Heart Rate inDe Novo Patients with Parkinson’s Disease - Kyum-Yil Kwon1,2, Seon Jong Pyo1, Hye Mi Lee1, Woo-Keun Seo1, Seong-Beom Koh1
-
Journal of Movement Disorders 2016;9(3):144-151.
DOI: https://doi.org/10.14802/jmd.16012
Published online: September 21, 2016
1Department of Neurology and Parkinson’s Disease Centre, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
2Department of Neurology, Soonchunhyang University Gumi Hospital, Soonchunhyang University School of Medicine, Gumi, Korea
- Corresponding author: Seong-Beom Koh, MD, PhD, Department of Neurology and Parkinson’s Disease Centre, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 08308, Korea / Tel: +82-2-2626-3169 / Fax: +82-2-2626-1257 / E-mail: parkinson@korea.ac.kr
Copyright © 2016 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- We sought to identify whether the characteristics of long-term visit-to-visit blood pressure (BP) and heart rate (HR) are related to baseline cognitive profiles in, Parkinson’s disease (PD).
-
Methods
- We selected drug-naïve PD patients who visited our hospital at least 10 times with a baseline assessment of the Seoul neuropsychological battery. BP and HR were measured at each visit, and the variability of the systolic BP/diastolic BP (DBP) and HR was derived from the parameters of serial 10 office visits. Mild cognitive impairment (MCI) in PD patients was determined according to the proposed criteria with a cut-off value of z-score ≤ -2.
-
Results
- Forty-seven patients with PD (mean follow-up duration = 22.3 months) were enrolled in the study. Compared with non-MCI PD patients, MCI PD patients revealed a significant increase in HR and/or variability in DBP.
-
Conclusion
- This exploratory study showed that baseline cognition in drug-naïve PD patients might be related to the visit-to-visit variability of DBP and/or HR.
- Subjects
- We retrospectively selected forty-seven de novo patients with PD by reviewing medical records at the Parkinson’s Disease Centre in Korea University Guro Hospital from January 2009 to March 2011. The inclusion criteria were as follows: 1) patients were diagnosed with de novo PD according to the UK brain bank criteria [22]; 2) 3-T MRI scanning, including axial fluid attenuated inversion recovery images, and a full battery of neuropsychological tests were conducted in the same subject; 3) we excluded patients with dementia or moderate to severe ischemic or other structural lesions, based on the review of their brain MRI images; 4) all patients had visited our clinic at least 10 times; and 5) the existence of non-motor symptoms including rapid eye movement sleep behavior disorder (RBD)-like symptoms and subjective hyposmia was analyzed. The present study was approved by the Institutional Review Board of the Korea University Guro Hospital (KUGH IRB #14236).
- Neuropsychological assessment and PD-MCI
- The neuropsychological tests were administered in a constant protocol. The Seoul neuropsychological battery (SNSB) was performed on all patients with PD. The Korean version of the Mini-Mental Status Examination and the Geriatric Depression Scale was assessed in the same session. Because normative data of the SNSB from healthy Korean subjects were available, age-, gender-, and education-matched percentiles were obtained from the raw score of each test and thereby converted into z-scores. According to the task force guidelines from the movement disorder society [23], each test of the SNSB was categorized into five cognitive domains: attention and working memory, executive function, language, memory, and visuospatial function (Supplementary Table 1 in the online-only Data Supplement). MCI in patients with PD was defined according to the recommended Movement Disorder Society task force criteria [23]. Impaired cognition in each test was determined with a cut-off value of 2 standard deviation (SD) below the appropriate norms. The subtypes were classified as follows: single-domain PD-MCI (amnestic or nom-amnestic type) was derived from impairments on two tests within one single domain, with the other unimpaired, and multiple-domain PD-MCI was defined by abnormalities on at least one test in two or more domains. Because only one test was available in the SNSB for language and visuospatial function, abnormality on one test within such a domain was considered the fulfillment of a single-domain MCI pattern in the present study.
- BP and HR measurements and visit-to-visit variability
- Clinic BP and HR were measured in the sitting position after resting for approximately 5 to 10 minutes using a validated automatic oscillometric device (Model FT-500R, JAWON Medical Co. Ltd., Daejeon, Korea) at each visit. Overall, 10 values of BP and HR were obtained in 10 serial consecutive visits. The mean, maximum, and minimum values were calculated from the measured parameters, including SBP, diastolic BP (DBP), and HR. For the visit-to-visit variability, both the SD and coefficient of variation (CV) were assessed. CV was derived from the ratio of SD to the mean (CV = SD / mean × 100%).
- Statistical analyses
- Group comparisons for non-MCI vs. MCI were conducted using Student’s t-test for continuous variables and Fisher’s exact test for categorical data, respectively. Analysis of covariance (ANCOVA) was performed using Bonferroni correction for visit-to-visit BP and HR parameters between PD with and without MCI. To predict MCI in de novo PD patients, logistic regression analysis was conducted. Because univariate analysis showed only one significant variable, multivariate analysis could not be performed. Correlation studies between cognitive tests and variability of BP and HR were analyzed using Pearson’s correlation coefficient. A p value < 0.05 was defined as statistically significant. Each statistical analysis was performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA).
MATERIALS & METHODS
- Cognitive profiles of MCI in de novo patients with PD
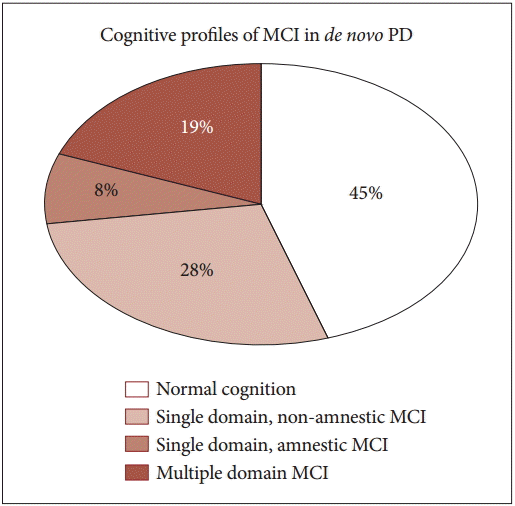
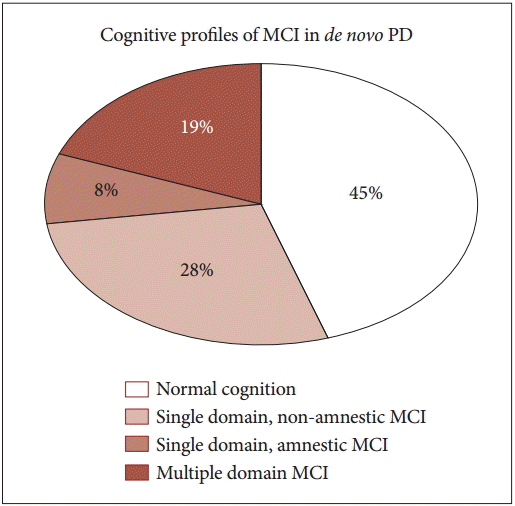
- Based on neuropsychological data described in Supplementary Table 1 (in the online-only Data Supplement), subtype classification was performed in the study population. The cognitive profiles of patients were distributed as shown in Figure 1: 45% (21 of 47 patients) with normal cognition, 28% (13 of 47) with non-amnestic single-domain MCI, 19% (9 of 47) with multiple-domain MCI, and 8% (4 of 47) with amnestic single-domain MCI.
- Clinical characteristics between non-MCI and MCI in de novo PD
- Baseline examinations included age, gender, duration of education, parkinsonism motor symptoms, disease duration at the first and last visits, daily equivalent dose of levodopa at the last visit, body weight, height, current smoking status, and past medical history (diabetes mellitus, hypertension, previous stroke, coronary disease, other heart disease, and chronic kidney disease). Table 1 summarizes the results of those characteristics in the comparison between the non-MCI and MCI groups. Only the level of education revealed a tendency of having shorter durations of education in PD MCI compared with PD non-MCI (mean 6.7 years vs. mean 9.1 years, p = 0.082). The rest of the clinical characteristics did not show any differences between the groups.
- Visit-to-visit BP and HR parameters between non-MCI and MCI in de novo PD
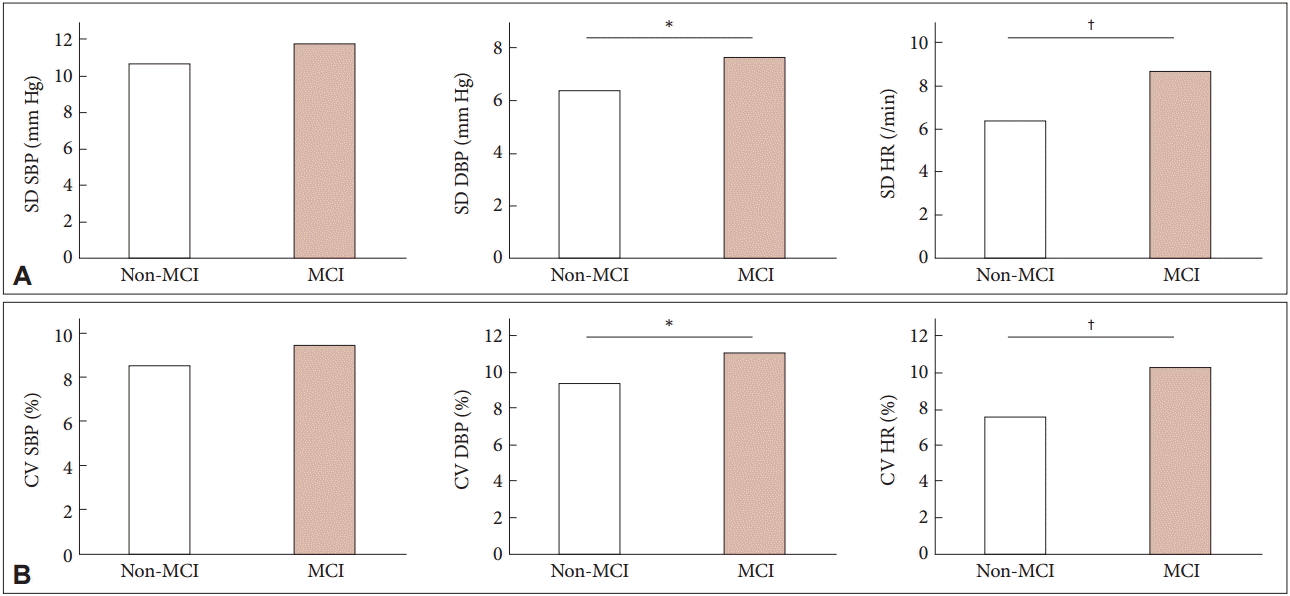
- For the profiles of visit-to-visit SBP, DBP, and HR, the comparisons between the non-MCI and MCI group are shown in Table 2. The primary parameters of SBP, DBP, and HR including mean, maximum, and minimum vales were not different between the groups. The visit-to-visit variability of DBP and HR is shown in Table 3, Figure 2, and Supplementary Table 2 (in the online-only Data Supplement). ANCOVA in Table 3 exhibited not only a higher variability of visit-to-visit DBP (p = 0.042) but also a tendency of visit-to-visit HR variability (p = 0.078). As shown Figure 2, PD MCI patients revealed a tendency of higher variability of visit-to-visit DBP, compared with non-MCI patients (SD values of 7.6 ± 2.7 mm Hg vs. 6.4 ± 1.4 mm Hg, p = 0.057; CV values of 11.1 ± 4.0% vs. 9.4 ± 2.2%, p = 0.080, respectively). Moreover, the visit-to-visit HR variability was greater in patients with PD MCI (SD = 8.7 ± 4.2 mm Hg; CV = 11.1 ± 5.9%, respectively) than in those with PD non-MCI (SD = 6.4 ± 2.5 mm Hg, p = 0.027; CV = 8.0 ± 3.0%, p = 0.020, respectively). However, there was no difference in the visit-to-visit SBP variability between both of them.
- In addition, univariate logistic regression analysis was performed to predict MCI in de novo PD. As shown in Table 4, we found that visit-to-visit HR variability showed a significant risk factor for having MCI in de novo patients with PD.
- Additionally, because anti-hypertensive medication could affect the variability of BP or HR, we compared the hypertensive and non-hypertensive PD groups, as shown in Supplementary Table 3 (in the online-only Data Supplement). The BP and HR parameters, including variability, were not different between the groups.
- Correlations of cognitive test and visit-to-visit variability of BP or HR
- We conducted correlation analyses for each cognitive test of the SNSB and each variability parameter in Supplementary Table 2 (in the online-only Data Supplement). The visit-to-visit SBP or HR variability did not show any correlation with cognitive tests. However, the visit-to-visit DBP variability was connected with memory function. Specifically, the visit-to-visit DBP variability was strongly associated with verbal memory: one test displayed a significant negative correlation (r = -0.389, p = 0.007), and the other two tests revealed a tendency for a negative correlation (r = -0.283, p = 0.054; r = -0.273, p = 0.063, respectively). In addition, one test of the visual memory domain resulted in a negative correlation with visit-to-visit DBP variability (r = -0.316, p = 0.030).
- Visit-to-visit BP and HR parameters based on RBD-like symptoms or subjective hyposmia
- Approximately 28% (13 of 47) and 43% (20 of 47) of the patients reported subjective hyposmia. In Supplementary Table 4 (in the online-only Data Supplement), although the visit-to-visit variability of SBP and DBP showed no difference in subjective hyposmia, visit-to-visit HR variability was significantly increased in the presence of subjective hyposmia. In Supplementary Table 5 (in the online-only Data Supplement), none of the parameters of visit-to-visit variability of BP or HR were dependent on RBD-like symptoms.
RESULTS
- To our knowledge, this is the first study to demonstrate the relationship between cognition and the visit-to-visit variability of BP and HR in de novo PD patients. The current study showed that MCI was 55% (26 of 47 patients) in drug-naïve PD patients (Figure 1). This was a relatively higher proportion of PD with MCI compared to previous studies, which reported MCI values of up to 42.5% in de novo PD populations [1,24]. This high prevalence of MCI in newly diagnosed PD might have been derived from the following: 1) at the beginning of enrollment of de novo PD patients, a total of 54 patients revealed no or minimal structural lesions in brain MRI. However, seven patients without MCI were excluded because they were all lost to follow-up before 10 serial office visits. In contrast, all PD patients with MCI and 10 serial visits were included. Thus, the prevalence of MCI in the current study was approximately 48% (26 of the 54 de novo patients). 2) Our Korean cohort revealed a female preponderance, which might affect the prevalence of MCI. In general, the PD population showed a male preponderance. One Korean study with an early PD population revealed a female preponderance in both the MCI and dementia subgroups. However, our point of view remains uncertain and thus should not be generalized.
- We found that clinical characteristics, including age, gender, and level of education, did not affect the cognitive impairment in patients with de novo PD (Table 1), although only PD with MCI showed a tendency (p = 0.082) of longer duration of education compared with PD without MCI. Disease severity, including total motor score and Hoehn and Yahr stage, depression score, comorbidities, and last PD medications, were not significantly different between the two groups. Moreover, our results showed that the BP or HR parameters, including variability, were not dependent on the usage of antihypertensive agents (Supplementary Table 3 in the online-only Data Supplement). Collectively, our results suggested that we could not predict the existence of MCI in early PD patients according to baseline clinical features.
- PD MCI showed significant differences in the visit-to-visit variability of HR and DBP, compared with PD non-MCI. However, the SBP variability was not different between the groups. Consistent with the literature [13], our findings showed that cardiovascular fluctuation was associated with cognitive impairment, even in the early stage of PD. According to neuropathological studies [25,26], α-synuclein aggregates were accompanied by neuronal cell loss in the sympathetic ganglia, indicating that cardiac sympathetic degeneration could lead to visit-to-visit variability in both BP and HR in PD. However, the mechanism of how visit-to-visit variability in BP or HR influence cognitive dysfunction remains unknown, especially in de novo patients with PD. As previously described, the BP variability might predict cognitive deterioration in the elderly or patients with Alzheimer’s dementia [19,20]. Two review articles reported that increased BP variability is related with vascular brain injury, including stroke or asymptomatic brain lesions [27,28]. Recently, Nagai and Kario [28] proposed the following hypothesis: arterial remodeling induced by high visit-to-visit BP variability might deregulate cerebral circulation and reduce cerebral blood flow; therefore, subsequent silent brain injury could influence cognitive impairment or deterioration. These previous studies demonstrated that cognitive dysfunction was associated with the visit-to-visit variability of SBP rather than that of DBP, while we found that cognitive impairment in PD population was related to DBP and HR variability, apart from SBP variability. Therefore, our results suggested that the pathophysiologic mechanism of BP or HR variability in PD might differ from that in stroke or Alzheimer’s disease.
- We correlated each cognitive test with the visit-to-visit variability of SBP, DBP, and HR in Supplementary Table 2 (in the online-only Data Supplement). The DBP variability in PD subjects not only showed a significant association with recognition memory in verbal as well as visual tests but also revealed a negative correlation with verbal and visual recall memory. One group reported that both SBP and DBP variability showed associations with several cognitive deficits, including immediate and delayed memory function, in the elderly [29]. Interestingly, the study revealed that hippocampal atrophy was related to the increased visit-to-visit variability in SBP as well as DBP. Thus, there is a possibility that the BP variability might be associated with the volume of the hippocampus.
- In addition, we analyzed whether other non-motor symptoms, including RBD-like symptoms and subjective hyposmia, could affect visit-to-visit BP or HR, respectively. Neither RBD-like symptoms nor subjective hyposmia was related to MCI in patients with PD (data not shown). HR variability was associated with subjective hyposmia but not with RBD-like symptoms (Supplementary Table 1 and 5 in the online-only Data Supplement). Oka et al. [30] previously revealed that olfactory dysfunction might be related to cardiovascular dysfunction in PD. Taken together, our results suggest that a common neurodegenerative pathway or network may be involved in both olfactory and cardiovascular systems in PD.
- The present study had several potential short-comings. First, our study was a retrospectively designed study with a relatively small sample number, and the baseline neuropsychological assessment and BP/HR variability showed some associations in our study population. However, they could be separate manifestations of the neurodegenerative progression of PD, and our results should be interpreted with caution. Second, although we tried to minimize the effect of vascular burden in the brain by only including patients with no or mild ischemic lesions on their MRI scans, we did not investigate the relationship between vascular lesions and cognitive impairment in the current study. Therefore, we could not rule out the possibility that vascular lesions might affect cognitive impairment in patients with PD. It remains unknown whether subtle ischemic changes could affect our observations. Third, we could not adequately control unknown confounding factors, including visit time. Conversely, this indirectly reflects real clinical settings in the treatment of PD. Fourth, other non-motor symptoms, including RBD-like symptoms and subjective hyposmia, were not checked by using objective assessment tools.
- In conclusion, we showed that the visit-to-visit DBP and HR variability might be related to cognitive dysfunction in de novo PD patients. Additionally, the visit-to-visit HR variability was associated with subjective hyposmia, regardless of cognitive impairment. The current study suggests that clinicians should pay more attention to the office BP and HR of patients with PD in clinical practice. Because this study is a preliminary study, well-designed studies with large sample sizes will be required to uncover the detailed relationship between non-motor symptoms, including cognitive dysfunction and cardiovascular variability, in patients with PD.
DISCUSSION
Supplementary Materials

Data with continuous variable are presented as mean ± standard deviation. Student t-test for continuous variable and Fisher’s exact test for categorical variable. PD: Parkinson’s disease, MCI: mild cognitive impairment, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, BMI: body mass index, K-MMSE: Korean version of Mini-Mental Status Examination, LEDD: levodopa equivalent daily dose, ARB: angiotensin receptor blocker, ACE: angiotensin converting enzyme, UPDRS: Unifed Parkinson’s Disease Rating Scale.
Data are presented as mean ± standard deviation. Data are adjusted for age, gender, UPDRS-motor, Depression Scale, MMSE, and levodopa equivalent daily dose. PD: Parkinson’s disease, MCI: mild cognitive impairment, ANCOVA: analysis of covariance, CV: coefficient of variation, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, NS: not significant, UPDRS: Unifed Parkinson’s Disease Rating Scale, MMSE: Mini-Mental Status Examination.
PD: Parkinson’s disease, MCI: mild cognitive impairment, BMI: body mass index, UPDRS: Unifed Parkinson’s Disease Rating Scale, K-MMSE: Korean version of Mini-Mental Status Examination, SD: standard deviation, CV: coefficient of variation, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate.
- 1. Poletti M, Emre M, Bonuccelli U. Mild cognitive impairment and cognitive reserve in Parkinson’s disease. Parkinsonism Relat Disord 2011;17:579–586.ArticlePubMed
- 2. Poletti M, Frosini D, Pagni C, Baldacci F, Nicoletti V, Tognoni G, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 2012;83:601–606.ArticlePubMed
- 3. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844.ArticlePubMed
- 4. Takatsu H, Nishida H, Matsuo H, Watanabe S, Nagashima K, Wada H, et al. Cardiac sympathetic denervation from the early stage of Parkinson’s disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med 2000;41:71–77.PubMed
- 5. Umemura A, Oeda T, Hayashi R, Tomita S, Kohsaka M, Yamamoto K, et al. Diagnostic accuracy of apparent diffusion coefficient and 123I-metaiodobenzylguanidine for differentiation of multiple system atrophy and Parkinson’s disease. PLoS One 2013;8:e61066.ArticlePubMedPMC
- 6. Goldstein DS. Orthostatic hypotension as an early finding in Parkinson’s disease. Clin Auton Res 2006;16:46–54.ArticlePubMed
- 7. Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension 1998;31:780–786.ArticlePubMed
- 8. Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology 1998;51:986–993.ArticlePubMed
- 9. Prince MJ, Bird AS, Blizard RA, Mann AH. Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council’s trial of hypertension in older adults. BMJ 1996;312:801–805.ArticlePubMedPMC
- 10. Mehrabian S, Duron E, Labouree F, Rollot F, Bune A, Traykov L, et al. Relationship between orthostatic hypotension and cognitive impairment in the elderly. J Neurol Sci 2010;299:45–48.ArticlePubMed
- 11. Guo H, Tabara Y, Igase M, Yamamoto M, Ochi N, Kido T, et al. Abnormal nocturnal blood pressure profile is associated with mild cognitive impairment in the elderly: the JSHIPP study. Hypertens Res 2010;33:32–36.ArticlePubMedPDF
- 12. Nicolini P, Ciulla MM, Malfatto G, Abbate C, Mari D, Rossi PD, et al. Autonomic dysfunction in mild cognitive impairment: evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS One 2014;9:e96656. ArticlePubMedPMC
- 13. Kim JS, Oh YS, Lee KS, Kim YI, Yang DW, Goldstein DS. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 2012;79:1323–1331.ArticlePubMedPMC
- 14. Howard SC, Rothwell PM. Reproducibility of measures of visit-to-visit variability in blood pressure after transient ischaemic attack or minor stroke. Cerebrovasc Dis 2009;28:331–340.ArticlePubMed
- 15. Joyce EF, Pedersen M, Tiong S, White-Brown SK, Paul A, Campbell SD, et al. Drosophila ATM and ATR have distinct activities in the regulation of meiotic DNA damage and repair. J Cell Biol 2011;195:359–367.ArticlePubMedPMC
- 16. Cuffe RL, Howard SC, Algra A, Warlow CP, Rothwell PM. Medium-term variability of blood pressure and potential underdiagnosis of hypertension in patients with previous transient ischemic attack or minor stroke. Stroke 2006;37:2776–2783.ArticlePubMed
- 17. Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010;375:895–905.ArticlePubMed
- 18. Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011;57:160–166.ArticlePubMed
- 19. Lattanzi S, Luzzi S, Provinciali L, Silvestrini M. Blood pressure variability predicts cognitive decline in Alzheimer’s disease patients. Neurobiol Aging 2014;35:2282–2287.ArticlePubMed
- 20. Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues JF, Richard-Harston S, et al. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimers Dement 2014;10(5 Suppl):S330–S337.ArticlePubMed
- 21. Nagai M, Hoshide S, Nishikawa M, Masahisa S, Kario K. Visit-to-visit blood pressure variability in the elderly: associations with cognitive impairment and carotid artery remodeling. Atherosclerosis 2014;233:19–26.ArticlePubMed
- 22. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184.ArticlePubMedPMC
- 23. Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356.ArticlePubMedPMC
- 24. Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, Firbank MJ, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 2014;82:308–316.ArticlePubMedPMC
- 25. Orimo S, Takahashi A, Uchihara T, Mori F, Kakita A, Wakabayashi K, et al. Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol 2007;17:24–30.ArticlePubMedPMC
- 26. Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 2008;131(Pt 3):642–650.ArticlePubMedPDF
- 27. Diaz KM, Tanner RM, Falzon L, Levitan EB, Reynolds K, Shimbo D, et al. Visit-to-visit variability of blood pressure and cardiovascular disease and all-cause mortality: a systematic review and meta-analysis. Hypertension 2014;64:965–982.ArticlePubMedPMC
- 28. Nagai M, Kario K. Visit-to-visit blood pressure variability, silent cerebral injury, and risk of stroke. Am J Hypertens 2013;26:1369–1376.ArticlePubMedPDF
- 29. Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ 2013;347:f4600.ArticlePubMed
- 30. Oka H, Toyoda C, Yogo M, Mochio S. Olfactory dysfunction and cardiovascular dysautonomia in Parkinson’s disease. J Neurol 2010;257:969–976.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Association between the blood pressure variability and cognitive decline in Parkinson's disease
Yi Xiao, Tianmi Yang, Lingyu Zhang, Qianqian Wei, Ruwei Ou, Yanbing Hou, Kuncheng Liu, Junyu Lin, Qirui Jiang, Huifang Shang
Brain and Behavior.2023;[Epub] CrossRef - Cardiovascular autonomic dysfunction is associated with executive dysfunction and poorer quality of life in progressive supranuclear palsy-Richardson’s syndrome
Peng Liu, Yueting Chen, Bo Wang, Sheng Wu, Leilei Zeng, Zhidong Cen, Dehao Yang, Haotian Wang, Xinhui Chen, Lebo Wang, Zhiyuan Ouyang, Wei Luo
Journal of Clinical Neuroscience.2022; 96: 147. CrossRef - Blood Pressure Variability and Cognitive Function: a Scoping Review
Nur Fazidah Asmuje, Sumaiyah Mat, Phyo Kyaw Myint, Maw Pin Tan
Current Hypertension Reports.2022; 24(10): 375. CrossRef - Associations of cognitive dysfunction with motor and non-motor symptoms in patients with de novo Parkinson’s disease
Kyum-Yil Kwon, Suyeon Park, Rae On Kim, Eun Ji Lee, Mina Lee
Scientific Reports.2022;[Epub] CrossRef - Blood pressure variability is related to faster cognitive decline in ischemic stroke patients: PICASSO subanalysis
Yerim Kim, Jae-Sung Lim, Mi Sun Oh, Kyung-Ho Yu, Ji Sung Lee, Jong-Ho Park, Yong-Jae Kim, Joung-Ho Rha, Yang-Ha Hwang, Sung Hyuk Heo, Seong Hwan Ahn, Ju-Hun Lee, Sun U. Kwon
Scientific Reports.2021;[Epub] CrossRef - The correlation of blood pressure variability and cognitive function in hypertension patients: A meta‐analysis
Xiaojie Jin, Yi Lu, Peng Zhao
International Journal of Clinical Practice.2021;[Epub] CrossRef - Burden and correlates of cognitive impairment among hypertensive patients in Tanzania: a cross-sectional study
Pedro Pallangyo, Zabella S. Mkojera, Makrina Komba, Lucy R. Mgopa, Smita Bhalia, Henry Mayala, Salma Wibonela, Nsajigwa Misidai, Happiness J. Swai, Jalack Millinga, Ester Chavala, Peter R. Kisenge, Mohamed Janabi
BMC Neurology.2021;[Epub] CrossRef - Backward Gait is Associated with Motor Symptoms and Fear of Falling in Patients withDe NovoParkinson's Disease
Kyum-Yil Kwon, Suyeon Park, Hye Mi Lee, Young-Min Park, Jinhee Kim, Jaehwan Kim, Seong-Beom Koh
Journal of Clinical Neurology.2019; 15(4): 473. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite