Gender Differences in Age-Related Striatal Dopamine Depletion in Parkinson’s Disease
Article information
Abstract
Objective
Gender differences are a well-known clinical characteristic of Parkinson’s disease (PD). In-vivo imaging studies demonstrated that women have greater striatal dopamine transporter (DAT) activity than do men, both in the normal population and in PD patients. We hypothesize that women exhibit more rapid aging-related striatal DAT reduction than do men, as the potential neuroprotective effect of estrogen wanes with age.
Methods
This study included 307 de novo PD patients (152 men and 155 women) who underwent DAT scans for an initial diagnostic work-up. Gender differences in age-related DAT decline were assessed in striatal sub-regions using linear regression analysis.
Results
Female patients exhibited greater DAT activity compared with male patients in all striatal sub-regions. The linear regression analysis revealed that age-related DAT decline was greater in the anterior and posterior caudate, and the anterior putamen in women compared with men; we did not observe this difference in other sub-regions.
Conclusions
This study demonstrated the presence of gender differences in age-related DAT decline in striatal sub-regions, particularly in the antero-dorsal striatum, in patients with PD, presumably due to aging-related decrease in estrogen. Because this difference was not observed in the sensorimotor striatum, this finding also suggests that women may not have a greater capacity to tolerate PD pathogenesis than do men.
Gender differences are a well-known clinical characteristic of Parkinson’s disease (PD) [1]. PD occurs more often in men than in women: a meta-analysis showed an increased relative risk of 1.5 in men [2]. Age at PD onset is later in women than in men [3,4], and partly correlates with fertile life span in women [3]. Clinically, women with PD exhibit less severe PD motor features, and show greater levodopa responses with more severe levodopa-induced dyskinesia [5,6]. Female sex hormones (i.e., estrogen) are thought to play an important role in the gender differences observed in PD [7]. These gender differences suggest a beneficial influence of estrogens against the development and progression of PD.
Women also show higher striatal dopamine transporter (DAT) activity than do men, both in the normal population and in patients with PD [3,8-11]. This gender-related DAT difference is more prominent in the caudate than in the putamen [8]. In addition, striatal DAT activity declines with aging, both in the normal population [8-13] and in patients with PD [8,11]. The age-related decline in DAT activity mainly impacts the striatum in controls [8,11,14], and the caudate in patients with PD [12]. If greater DAT activity in women is related to the potential neuroprotective effects of estrogen, age-related DAT decline may be greater in women than in men, as the effects of estrogen wane with aging. Therefore, in this study, we investigated gender differences in the degree and pattern of aging-related striatal DAT decline in patients with PD.
MATERIALS & METHODS
Patients
Study subjects were selected from the Yonsei Parkinson Center database (patient sample collected from March 2009 to June 2013) and fulfilled the following selection criteria: 1) drug-naive PD patients, 2) who underwent DAT imaging, using [18F] N-(3-fluoropropyl)-2β-carbon ethoxy-3β-(4-iodophenyl) nortropane positron emission tomography (FP-CIT-PET) scans, and 3) who had intact cognitive function (Mini-Mental Status Examination score of 24 or higher). In these patients, PD was diagnosed according to the clinical criteria of the United Kingdom PD society Brain Bank [15], the presence of appropriate DAT uptake defects on FP-CIT PET scans [16], and the presence of anti-Parkinson drug response during follow-up (i.e., after 6 or more months of PD medication administration). Part III of the Unified Parkinson Disease Rating Scale (UPDRS-motor) was used to assess PD severity in each patient at the time of 18F-FP-CIT PET image acquisition. The ethics committee of our hospital reviewed and approved this study.
PET-CT image acquisition
To assess striatal dopamine depletion, we obtained DAT scans using 18F-FP-CIT with a GE Discovery STe (DSTE) PET-CT scanner (GE Healthcare Technologies, Milwaukee, WI, USA). Each patient fasted for 6 hours before receiving an intravenous injection of 5 mCi (185 MBq) 18F-FP-CIT. Ninety minutes after the injection, DAT images were obtained in 3 dimensional (3D) mode during a 20-minute session. Post hoc 3D Gaussian smoothing with a 2.3-mm full-width half-maximum was performed. For localization and attenuation correction, CT images were acquired at 120 KVp and 380 mAs after PET scanning.
Quantitative analysis of the PET image data
Quantitative analyses of FP-CIT PET data were performed according to a previously published procedure [16]. Images were processed using SPM8 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, London, UK) with MATLAB 2013a for Windows (Math-Works, Natick, MA, USA). Quantitative analyses were based on volumes of interest (VOIs), which were defined based on a template in standard space. All reconstructed PET images were spatially normalized to the Montreal Neurological Institute template space using a standard FP-CIT PET template, as described previously [16]. Twelve VOIs of both striatal sub-regions and one occipital VOI were drawn on a co-registered spatially normalized single T1 MRI and a FP-CIT PET template image using MRIcro version 1.37 (Chris Rorden, Columbia, SC, USA). DAT activity in each VOI was estimated as the surrogate of non-displaceable binding potential, defined as follows: [mean standardized uptake value (SUV) of the striatal sub-regions VOI - mean SUV of the occipital VOI] / mean SUV of the occipital VOI [17]. Mean DAT activities of bilateral striatal sub-regions were used in the statistical analyses.
Statistical analysis
Data are expressed as means ± SDs. Unpaired t-tests were used to compare clinical and DAT data between genders. An analysis of covariance with adjustment for age of onset was conducted to identify disparities in DAT activity and inter-sub-regional ratios (ISRs) between genders. General linear model and linear regression analyses controlled for age of onset, gender, disease duration, and UPDRS-motor scores, were performed to determine differences in age-related decline of DAT activity between genders. SPSS Statistics 20 (IBM, Armonk, NY, USA) was used to perform all statistical analyses. p-values < 0.05 were regarded as statistically significant.
RESULTS
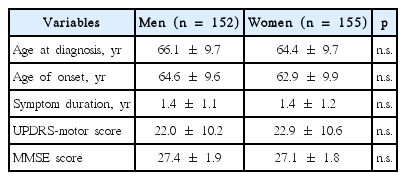
A total of 307 patients (152 men and 155 women) were included in the data analysis. The mean age of onset was 63.8 ± 9.8 (range, 37.0–85.4), the mean symptom duration was 1.4 ± 1.1 years, and the mean UPDRS-motor score was 22.4 ± 10.4. Baseline clinical characteristics are summarized in Table 1. All clinical variables were comparable between genders.
Striatal sub-regional DAT activities and ISRs are shown in Table 2. Female patients exhibited greater DAT activities in all striatal sub-regions than did male patients. Female patients also had higher ISRs in the anterior caudate/posterior putamen and in the posterior caudate/posterior putamen: the ISRs were comparable in other striatal sub-regions.
Female patients exhibited greater age-related decline in DAT activity in all striatal sub-regions except for the posterior putamen (Figure 1). After controlling for the influence of age of onset, the age-related decline in DAT activity was still significantly steeper in the anterior putamen and in the anterior and the posterior caudate. The DAT activity tended to be steeper in the ventral striatum in female versus male patients, but was comparable in the ventral and the posterior putamen between genders (Table 3). The same analysis was performed after controlling the influence of age of PET scan, instead of age of onset, and yielded similar results (Supplementary Table 1 in the online-only Data Supplement).

Scatterplots showing the age of onset and dopamine transporter (DAT) activity in the striatal sub-regions. A: Anterior caudate. B: Posterior caudate. C: Ventral striatum. D: Anterior putamen. E: Posterior putamen. F: Ventral putamen. In all sub-regions, women (closed triangle) exhibited higher DAT activities than did men (open circle). Women (dotted line) showed a more rapid age-related DAT decline in the anterior caudate (A), posterior caudate (B), and the anterior putamen (D), compared with men (solid line).
DISCUSSION
This study demonstrated that female patients showed greater DAT activity in all striatal sub-regions than did male patients. However, in this study, female patients also exhibited a more rapid age-related decline in DAT activity than did male patients, particularly in striatal sub-regions other than the sensorimotor striatum. We used age of onset in the statistical analyses performed in this study, because we primarily focused the influence of gender on striatal DAT activity at the time of PD onset (i.e., we assumed that the protective effect of female sex hormone could affect PD onset). In the analyses controlling for age of PET scans instead of age of onset showed similar results. Clinical variables were comparable between genders in this study, a finding that is in contrast to previous studies that reported later PD onset and less motor deficits in women than men with PD [3-6]. In this study, male patients exhibited later PD onset of 1.7 years than female patients, but this difference did not attain statistical significance.
Our finding of greater striatal DAT activity in women compared with men is consistent with a number of previous studies. In healthy individuals, women have significantly greater striatal DAT activity than do men [9,18,19]. A previous study that evaluated postsynaptic dopamine receptor activity using 11C-raclopride PET also reported a similar finding in healthy individuals [20]. In PD, two studies using 123I-FP-CIT single photon emission computed tomography (SPECT) revealed higher striatal DAT binding in female than male patients [3,8]. Haaxma et al. [3] reported that women had 16% higher striatal FP-CIT binding than men at symptom onset, while Kaasinen et al. [8] showed that women had greater DAT binding in the caudate, but not in the putamen. Because of the limited spatial resolution of the SPECT scan, they could not measure DAT activity in each striatal sub-region. We were able to subdivide striatal DAT activity into six sub-regions due to the superior spatial resolution of PET scanning, and found that greater DAT activity was present in all striatal sub-regions in women compared with men. Furthermore, we then analyzed ISRs, and found that women had higher ISRs in the anterior caudate/posterior putamen and in the posterior caudate/posterior putamen. Higher ISRs in women in this study may represent either greater DAT activities in the anterior and posterior caudate relative to those in the posterior putamen or less DAT activities in the posterior putamen relative to those in the anterior and posterior caudate. Because women exhibited greater DAT activities in all striatal sub-regions than did men, we could disregard the latter possibility. Thus, these findings suggest that the gender-related DAT activity difference is greater in the caudate compared with the putamen, which is partly compatible with the findings of Kaasinen et al. [8].
The age-related striatal DAT decline observed in this study was also demonstrated in a number of previous studies [9-13]. However, the effect of gender on striatal DAT activity has been controversial. Lavalaye et al. [9] observed that the effect of gender on 123I-FP-CIT binding ratios was not related to age, while Wong et al. [10] demonstrated that DAT activity significantly differed between genders in their young-to-middle age group (< 60 years), but not in the elderly age group. The present study supports Wong et al.’s findings, and illustrates that gender differences in age-related striatal DAT decline is also present in patients with PD. Furthermore, this study also demonstrated that gender differences in age-related DAT decline was more prominent in the other striatal sub-regions than in the ventral and posterior putamen. The striatum is organized into sensorimotor, associative, and limbic regions: the motor and premotor cortex project to the posterior and ventral putamen, and control sensorimotor function (i.e., the sensorimotor stratum) [21,22]. Because PD pathology preferentially affects the sensorimotor striatum, producing the motor deficits of PD, our findings suggest that PD-related pathology in the sensorimotor striatum contributes to the loss of gender effects on age-related DAT decline. Lee et al. [11] reported that age-related decline in DAT binding was preserved in the caudate, but was lost in the putamen of patients with PD. The authors suggested that differential age effects in the parkinsonian striatum likely reflect the superimposition of disease-driven compensation on the aging effect. Thus, our results may represent the superimposition of PD-driven influence on the gender effect.
Our study demonstrated that women had greater striatal DAT activity than did men, and women exhibited a more rapid decline of this activity with aging: the reason for this is unclear. A previous review suggested that the beneficial effects of estrogen contributed to this phenomenon [7]. Animal experiments showed that estrogen protects dopaminergic neurons against various neurotoxins that induce PD-like symptoms, by promoting survival factors, activating signaling pathways, interacting with growth factors, and exerting an anti-aggregation effect on alpha-synuclein [7,23-25]. Clinically, hormone replacement therapy with estrogen lowers the risk of PD and ameliorates motor deficits in PD [26-28]. The development and severity of PD are associated with lower lifetime estrogen exposure or shorter fertile life span [29,30]. These observations suggest that fertile life with sufficient estrogen maintenance in women has a key role in protecting striatal dopaminergic neurons against aging-related processes, and in maintaining a greater number of dopaminergic neurons during fertile life compared with men. This protective effect of estrogen may wane with aging (i.e., at the end of fertile life), which results in a more rapid age-related decline in dopaminergic neurons in women than in men, as observed in this study. Thus, the lack of a gender effect in age-related DAT decline in the sensorimotor striatum in this study suggests that the effect of estrogen in women can protect dopaminergic neurons against age-related processes, but not against PD-related pathogenesis.
In this study, measurements of striatal DAT activity and UPDRS-motor scores were conducted in drug-naive patients, sufficiently excluding the influence of any PD medication on the present results. A programmed measure of striatal DAT activities could minimize the inter-rater and intra-rater variability. However, this study had few notable limitations. Primarily, this study could not completely exclude the possibility of atypical parkinsonian syndromes due to the enrollment of early-stage patients with PD and a relatively short follow-up. Secondly, the lack of data from healthy controls precluded a comparison of the present results with normal age-related changes in striatal DAT activity. Thus, a further study that includes a sufficient number of healthy controls is warranted to address whether the findings observed in the present study are PD-specific or not.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Supplementary Material
The online-only Data Supplement is available with this article at http://dx.doi.org/10.14802/jmd.15031.
jmd-8-3-130-supple1.pdf