Articles
- Page Path
- HOME > J Mov Disord > Volume 7(1); 2014 > Article
-
Case Report
Suppression of Myoclonus in Corticobasal Degeneration by Levetiracetam - Jae Wook Cho, Jae Hyeok Lee
-
Journal of Movement Disorders 2014;7(1):28-30.
DOI: https://doi.org/10.14802/jmd.14007
Published online: April 30, 2014
Department of Neurology, Pusan National University Yangsan Hospital, Yangsan, Korea
- Corresponding author: Jae Hyeok Lee, MD, PhD, Department of Neurology, Pusan National University Yangsan Hospital, 20 Geumo-ro, Mulgeumeup, Yangsan 626-770, Korea / Tel: +82-55-360-2453 / Fax: +82-55-360-2152 / E-mail: jhlee.neuro@pusan.ac.kr
• Received: August 2, 2012 • Revised: October 13, 2012 • Accepted: March 23, 2014
Copyright © 2014 The Korean Movement Disorder Society
ABSTRACT
- Myoclonus in corticobasal degeneration (CBD) has often been associated with severe and difficult to treat disabilities. Levetiracetam is a new antiepileptic agent with antimyoclonic effects. Herein, we present a 72-year-old woman with clinically probable CBD and with spontaneous rhythmic myoclonus in the right foot, which was markedly ameliorated through treatment with levetiracetam. The effect of levetiracetam was associated with the decreased amplitude of enlarged cortical somatosensory evoked potentials. This result suggests that the antimyoclonic effect of levetiracetam might be mediated through the suppression of increased cortical excitability.
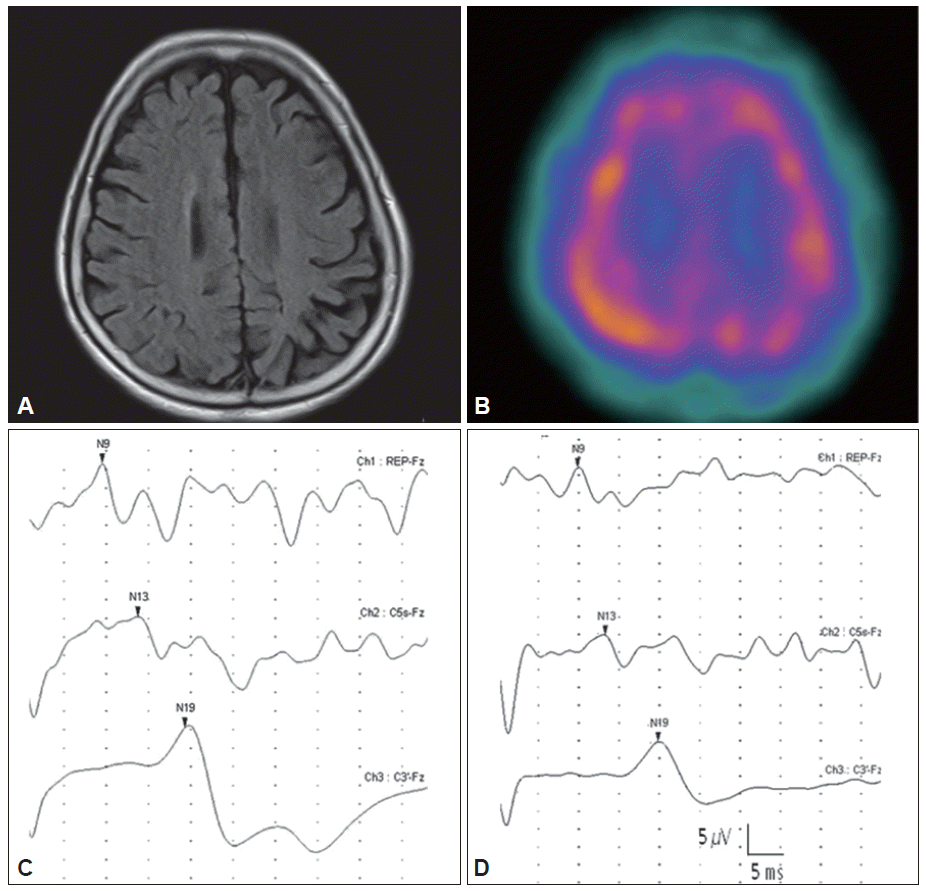
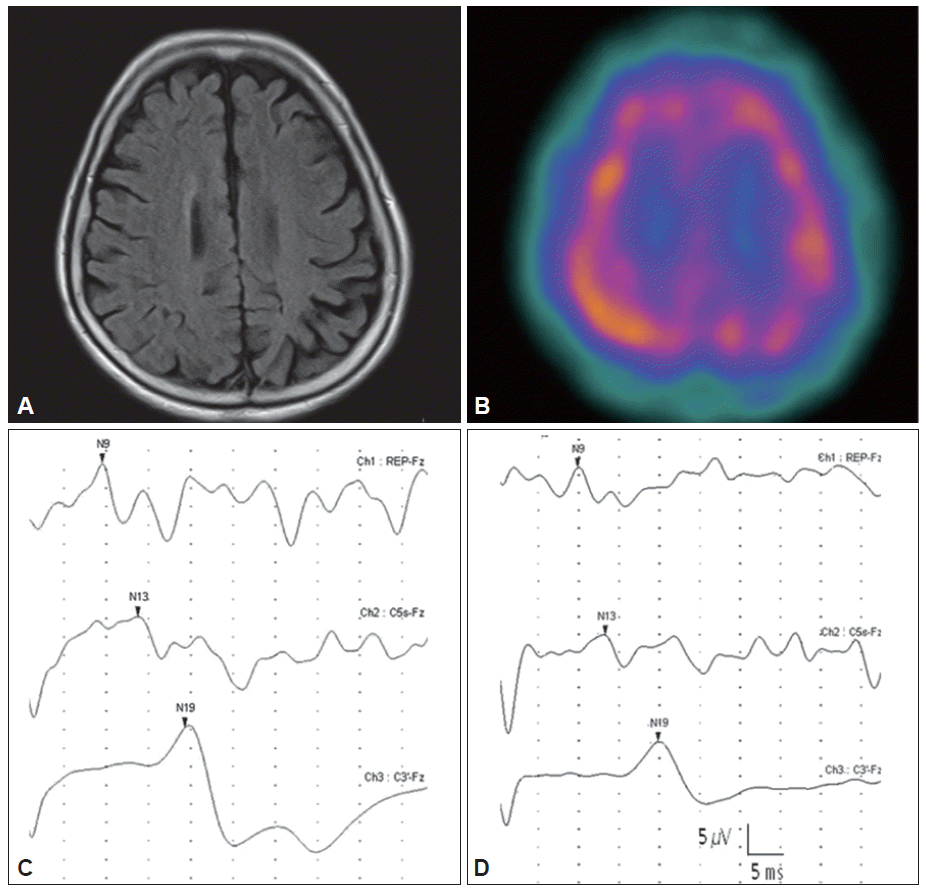
- This 72-year-old woman noticed difficulty walking and a tendency to fall at the age of 67 years, when she also developed jerking in her right leg and foot when attempting to use the leg. After 2 years, rhythmic jerking in her right foot spontaneously occurred, further interfering with voluntary movements of the right leg. The woman gradually lost complete control of limb and was unable to walk at 3 years after the onset of these symptoms. During this same time period, the woman also observed a jerky action tremor in her right fingers. Neurological examination at 5 years after onset showed generalized bradykinesia and rigidity predominating in the right limbs. The right foot was held in a fixed dystonic posture with plantar flexion and inversion. Marked spontaneous repetitive myoclonus in the right leg was observed, and this condition was exacerbated through sensory stimuli and actions (Video clip). The woman presented a milder jerky intention tremor in the right hand. Abnormal graphesthesia and ideomotor apraxia were observed in the right upper and lower limbs. The following laboratory examinations were not remarkable: cerebrospinal fluid analysis, spine MRI, spine electromyography, and electroencephalography. The brain MRI revealed asymmetric atrophy of the parietal and posterior regions of the frontal lobe, and this condition was more pronounced on the left (Figure 1A). The single photon emission computed tomography analysis showed asymmetric hypoperfusion in the atrophied regions (Figure 1B). Surface electromyography signals recorded in the right tibialis anterior and gastrocnemius muscles showed rhythmic repetitive trains of 25–50 ms discharges with simultaneous activation in agonist-antagonist pairs. The amplitudes of the N20 and P25 components of median somatosensory evoked potentials (right/left = 11.04/18.33 μV) revealed enlarged cortical waves (Figure 1C). After the ineffective administration of levodopa, valproic acid and clonazepam, levetiracetam was administered at an initial dose of 250 mg twice daily for two weeks. Subsequently, rhythmic resting and action myoclonus were markedly improved in the woman’s right foot (Video clip). The Unified Myoclonus Rating Scale (pre-treatment/2nd post-treatment week scores) was used to determine myoclonus at rest (right leg; 9/2) and myoclonus with action (right leg; 12/4); however, the fixed dystonic posturing in the foot remained unchanged. The action-induced jerky tremor in the upper limbs only modestly decreased. The amplitude of the enlarged N20-P25 component (right/left = 9.18/10.98 μV) was markedly reduced at four weeks after treatment (Figure 1D). The drug was well tolerated and non-sedating. Moreover, the clinical efficacy of levetiracetam treatment was maintained for 6 months during the follow-up period.
CASE
- Levetiracetam is a new antiepileptic agent that exerts antimyoclonic effects on various disorders. Indeed, this drug has been successfully used to treat post-hypoxic myoclonus, post-encephalitic myoclonus, 4 and other typical cortical myoclonus observed in progressive myoclonic epilepsy/ataxia.5 The effect of levetiracetam is mediated through binding to synaptic vesicle glycoprotein 2A.4–6 In studies concerning the effect of this drug treatment on transcranial magnetic stimulation parameters, levetiracetam decreased cortical excitability through the GABAergic mechanism.6
- A giant SEP, defined as a larger than 8.6 mV amplitude difference between the N20 and P25 peaks or a larger than 8.4 mV amplitude difference between the N33 and P25 peaks at the central cortical area, represents increased cortical neuronal excitability. 7 In the present study, the amplitude of the N20-P25 component was markedly enlarged on the affected side, while the amplitude of the P25- N33 component was not enlarged. Similar findings have been reported in CBD.1
- We also observed that the effect of levetiracetam was associated with a decrease in the amplitude of enlarged cortical SEPs. The amplitude of N20-P25 components involving the prominent myoclonic limb was markedly reduced up to 40% after treatment. This finding is consistent with previous results obtained in patients with chronic cortical myoclonus. 5 Levetiracetam significantly reduced the amplitude of N20-P25 components, whereas no significant difference was observed between the pre- and post-treatment P25-N30 amplitudes. Taken together, these results suggest that the antimyoclonic effect of levetiracetam treatment on CBD is similar to that of chronic cortical myoclonus, and this effect is mediated through the suppression of some aspects of increased cortical excitability.
DISCUSSION
- The video shows spontaneous, repetitive rhythmic myoclonus in the right leg, which was further exacerbated through action. The right foot was held in a fixed dystonic posture with plantar flexion and inversion. After two weeks of treatment, rhythmic resting and action myoclonus in the right foot were markedly improved. However, the fixed dystonic posturing in the foot remained unchanged.
LEGEND TO THE VIDEO
- This study was supported through a grant from the Pusan National University Yangsan Hospital (2011).
Acknowledgments
Acknowledgments
Figure 1Brain MRI showing the asymmetric atrophy of the parietal and the posterior region of the frontal lobe, and these complications were more pronounced on the left, as noted (A). The SPECT analysis showed asymmetric hypoperfusion in the atrophied regions (B). The amplitudes of the N20-P25 components revealed enlarged somatosensory evoked potentials in the central cerebral areas on the left (C: 18.33 μV). The amplitude of enlarged N20-P25 components was reduced approximately 40% at four weeks after treatment (D: 10.98 μV). SPECT: single photon emission computed tomography.

- 1. Thompson PD, Day BL, Rothwell JC, Brown P, Britton TC, Marsden CD. The myoclonus in corticobasal degeneration. Evidence for two forms of cortical reflex myoclonus. Brain 1994;117(Pt 5):1197–1207.ArticlePubMedPDF
- 2. Caviness JN. Parkinsonism & related disorders. Myoclonus. Parkinsonism Relat Disord 2007;13( Suppl 3):S375–S384.Article
- 3. Kovács T, Farsang M, Vitaszil E, Barsi P, Györke T, Szirmai I, et al. Levetiracetam reduces myoclonus in corticobasal degeneration: report of two cases. J Neural Transm 2009;116:1631–1634.ArticlePubMed
- 4. Krauss GL, Bergin A, Kramer RE, Cho YW, Reich SG. Suppression of post-hypoxic and post-encephalitic myoclonus with levetiracetam. Neurology 2001;56:411–412.ArticlePubMed
- 5. Striano P, Manganelli F, Boccella P, Perretti A, Striano S. Levetiracetam in patients with cortical myoclonus: a clinical and electrophysiological study. Mov Disord 2005;20:1610–1614.ArticlePubMed
- 6. Solinas C, Lee YC, Reutens DC. Effect of levetiracetam on cortical excitability: a transcranial magnetic stimulation study. Eur J Neurol 2008;15:501–505.ArticlePubMed
- 7. Shibasaki H, Yamashita Y, Neshige R, Tobimatsu S, Fukui R. Pathogenesis of giant somatosensory evoked potentials in progressive myoclonic epilepsy. Brain 1985;108(Pt 1):225–240.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Management Strategies for Atypical Parkinsonism
Vasilios C. Constantinides, Nikolaos Giagkou, Maria-Evgenia Brinia, Christos Koros, Leonidas Stefanis, Maria Stamelou
Current Treatment Options in Neurology.2024; 26(5): 169. CrossRef - Chinese nutraceuticals and physical activity; their role in neurodegenerative tauopathies
Abdullahi Alausa, Sunday Ogundepo, Barakat Olaleke, Rofiat Adeyemi, Mercy Olatinwo, Aminat Ismail
Chinese Medicine.2021;[Epub] CrossRef - Four-Repeat Tauopathies: Current Management and Future Treatments
Lawren VandeVrede, Peter A. Ljubenkov, Julio C. Rojas, Ariane E. Welch, Adam L. Boxer
Neurotherapeutics.2020; 17(4): 1563. CrossRef - Physiology-Based Treatment of Myoclonus
Ashley B. Pena, John N. Caviness
Neurotherapeutics.2020; 17(4): 1665. CrossRef - Pharmacological interventions in corticobasal degeneration: a review
Leonardo Caixeta, Victor de Melo Caixeta, Yanley Lucio Nogueira, Tales Alexandre Aversi-Ferreira
Dementia & Neuropsychologia.2020; 14(3): 243. CrossRef - Available and future treatments for atypical parkinsonism. A systematic review
Davide Vito Moretti
CNS Neuroscience & Therapeutics.2019; 25(2): 159. CrossRef - An Update on Myoclonus Management
Christine M. Stahl, Steven J. Frucht
Expert Review of Neurotherapeutics.2019; 19(4): 325. CrossRef - Corticobasal degeneration: key emerging issues
F. Ali, K. A. Josephs
Journal of Neurology.2018; 265(2): 439. CrossRef - Focal Predominant Forms of Posthypoxic Action Myoclonus
Carmen Gasca‐Salas, Anthony E. Lang
Movement Disorders Clinical Practice.2016; 3(4): 417. CrossRef - Progressive Supranuclear Palsy and Corticobasal Degeneration: Pathophysiology and Treatment Options
Ruth Lamb, Jonathan D. Rohrer, Andrew J. Lees, Huw R. Morris
Current Treatment Options in Neurology.2016;[Epub] CrossRef - An Update and Review of the Treatment of Myoclonus
Kelly Mills, Zoltan Mari
Current Neurology and Neuroscience Reports.2015;[Epub] CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite