Articles
- Page Path
- HOME > J Mov Disord > Volume 15(2); 2022 > Article
-
Original Article
Development of Clinical Milestones in Parkinson’s Disease After Bilateral Subthalamic Deep Brain Stimulation -
Jed Noel A. Ong1,3
 , Jung Hwan Shin1
, Jung Hwan Shin1 , Seungho Jeon1
, Seungho Jeon1 , Chan Young Lee1
, Chan Young Lee1 , Han-Joon Kim1
, Han-Joon Kim1 , Sun Ha Paek2
, Sun Ha Paek2 , Beomseok Jeon1
, Beomseok Jeon1
-
Journal of Movement Disorders 2022;15(2):124-131.
DOI: https://doi.org/10.14802/jmd.21106
Published online: May 26, 2022
1Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
2Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
3Department of Neurology, Jose R. Reyes Memorial Medical Center, Manila, Philippines
- Corresponding author: Beomseok Jeon, MD, PhD Department of Neurology, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea / Tel: +82-2-2072-2876 / Fax: +82-2-3672-7553 / E-mail: brain@snu.ac.kr
- Corresponding author: Sun Ha Paek, MD, PhD Department of Neurosurgery, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea / Tel: +82-2-2072-2350 / Fax: +82-2-744-8459 / E-mail: paeksh@snu.ac.kr
Copyright © 2022 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objective
- Deep brain stimulation of the subthalamic nucleus (STN-DBS) in Parkinson’s disease (PD) patients does not halt disease progression, as these patients will progress and develop disabling non-levodopa responsive symptoms. These features may act as milestones that represent the overall functionality of patients after DBS. The objective of this study was to investigate the development of clinical milestones in advanced PD patients who underwent bilateral STN-DBS.
-
Methods
- The study evaluated PD patients who underwent STN-DBS at baseline up to their last follow-up using the Unified Parkinson’s Disease Rating Scale and Hoehn and Yahr scale. The symptoms of hallucinations, dysarthria, dysphagia, frequent falls, difficulty walking, cognitive impairment and the loss of autonomy were chosen as the clinical milestones.
-
Results
- A total of 106 patients with a mean age of 47.21 ± 10.52 years at disease onset, a mean age of 58.72 ± 8.74 years at surgery and a mean disease duration of 11.51 ± 4.4 years before surgery were included. Initial improvement of motor symptoms was seen after the surgery with the appearance of clinical milestones over time. Using the moderately disabling criteria, 81 patients (76.41%) developed at least one clinical milestone, while 48 patients (45.28%) developed a milestone when using the severely disabling criteria.
-
Conclusion
- STN-DBS has a limited effect on axial and nonmotor symptoms of the PD patients, in contrast to the effect on motor symptoms. These symptoms may serve as clinical milestones that can convey the status of PD patients and its impact on the patients and their caregivers. Therefore, advanced PD patients, even those treated with bilateral STN-DBS, will still require assistance and cannot live independently in the long run.
- Participants
- PD patients in our movement disorder center who underwent DBS have been evaluated since March 7, 2005, according to a previously described prospective protocol [6]. The current study was a retrospective analysis of a prospectively collected dataset. We reviewed 118 PD patients who underwent bilateral STN-DBS surgery before December 31, 2009. Patients were excluded if they 1) had previous brain surgery, 2) had DBS removal, or 3) had less than 2 years of follow-up. The study protocol was approved by the SNUH Institutional Review Board and conformed to the principles of the Declaration of Helsinki (IRB#2009-044-1155). Patient consent was not required due to the retrospective nature of the study.
- Neurosurgical procedure
- All patients underwent STN-DBS implantation as previously described [7]. The stimulation settings and medications were adjusted individually to maintain the optimal clinical condition.
- Clinical evaluation
- The current study evaluated the patients at baseline up to their last follow-up, which referred to data obtained at the last recorded admission at the movement disorders center ward. The patients were evaluated in their best condition with both medication and stimulation in the on state or during the on-stimulation state only if the patient was not taking any anti-parkinsonian medication at the time of evaluation.
- The parkinsonian states of the patients were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) and the modified Hoehn and Yahr (H&Y) stages. The levodopa equivalent daily dosage (LEDD) of all medications at baseline was calculated, and the baseline cognitive status was assessed using the Korean version of the Mini-Mental State Examination (K-MMSE).
- The following symptoms were chosen as the clinical milestones: 1) hallucination, 2) dysarthria, 3) dysphagia, 4) frequent falls, 5) difficulty walking, 6) cognitive impairment, and 7) loss of autonomy. Two sets of criteria were used to describe the occurrence of milestones: moderately disabling criteria and severely disabling criteria (Table 1).
- The clinical milestones chosen for this study were previously described physical, medication-related and cognitive/psychiatric symptoms, which greatly affect the QoL of PD patients [8-10]. Since the benefit of STN-DBS in controlling motor symptoms is already well established, we focused more on the axial and nonmotor symptoms that are not effectively addressed by DBS and chose them as the key clinical milestones. The chosen symptoms, such as hallucinations, speech and swallowing difficulties, mobility and cognitive impairment, are irreversible once present. Even if some of these symptoms are present prior to surgery, they persist postoperatively, making them good indicators of a patient’s QoL.
- Data analysis
- Descriptive analysis was used in this study. The values are expressed as the mean ± standard deviaion, unless otherwise specified. Survival analysis, including Kaplan–Meier (KM) graphs and log rank analyses, was used to evaluate milestone development over time. The analysis was performed in all PD patients and separately for males and females. The level of significance was set at p < 0.05. All statistical analyses were performed with SPSS 23.0 (IBM Corp., Armonk, NY, USA).
MATERIALS & METHODS
- Demographic data
- A total of 106 patients (45 males and 61 females) were included in this study. The demographic data and clinical characteristics are shown in Table 2. The mean age at disease onset was 47.21 ± 10.52 years, and the mean disease duration before surgery was 11.51 ± 4.4 years. Clinical assessments at baseline and after DBS surgery showed improvements of the UPDRS scores and reductions in the LEDD postoperatively. However, the initial improvements progressively worsened in the subsequent follow-ups with a concomitant increase in the LEDD (Supplementary Table 1 in the online-only Data Supplement). The mean follow-up duration after surgery was 8.90 ± 3.31 years, and the mean disease duration at the last follow-up was 21.75 ± 5.64 years.
- Clinical milestones
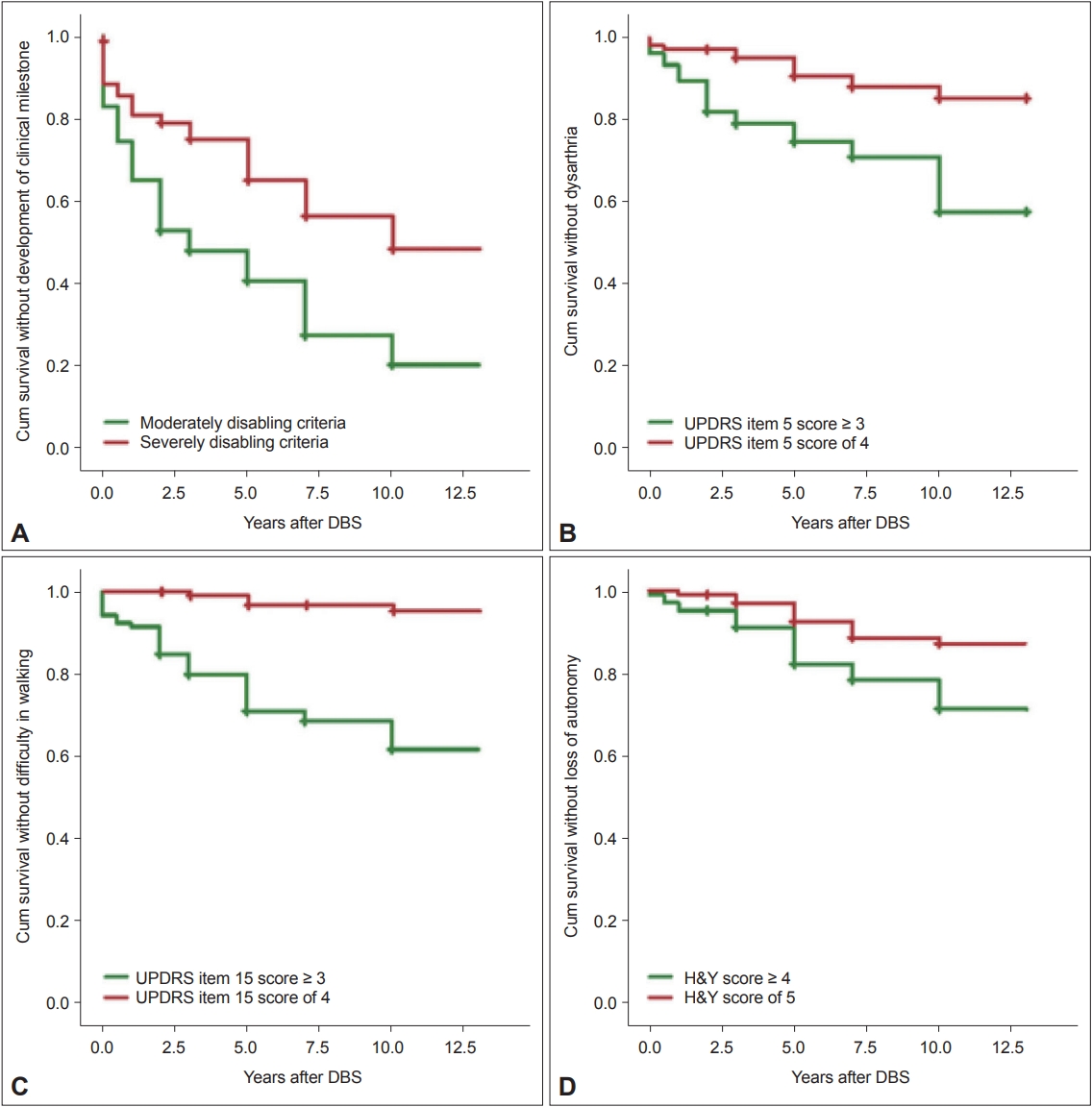
- Using the moderately disabling criteria, 81 out of the 106 patients (76.41%) developed at least one clinical milestone after STN-DBS, while 48 patients (45.28%) developed a milestone in the severely disabling criteria (Figure 1A). Before the surgery, 19 patients and 11 patients already had a clinical milestone according to the moderately and severely disabling criteria, respectively (Supplementary Tables 2 and 3 in the online-only Data Supplement). Using both criteria, the most frequent clinical milestone reported was frequent falls (Table 3).
- Using the moderately disabling criteria, two patients reported hallucinations at baseline. Over time, eight more patients developed persistent hallucinations. In total, 10 PD patients (9.43%) had hallucinations at their last follow-up. Using the severely disabling criteria, one patient had hallucinations at baseline, and only two more developed hallucinations over time, resulting in a total of three PD patients (2.83%) with hallucinations at their last follow-up.
- At baseline, four patients met the slurring of speech criterion in the moderately disabling criteria. Thirty-five additional patients were reported to have slurring of speech over time, with a total of 39 PD patients (36.79%) with dysarthria at their last follow-up. Using the severely disabling criteria, only two patients reported slurring of speech at baseline, and 11 more patients developed slurring of speech over time. A total of 13 PD patients (12.26%) reported developing dysarthria (Figure 1B).
- One patient reported difficulty swallowing at baseline when the moderately disabling criteria were used. Over time, 15 more patients developed difficulty swallowing, with a total of 16 PD patients (15.09%) having dysphagia at their last follow-up. However, when the severely disabling criteria were used, no patients had difficulty swallowing at their last follow-up.
- At baseline, fourteen PD patients reported frequent falls in the moderately disabling criteria. In the subsequent follow-ups, 43 more patients reported frequent falls. In total, 57 patients (53.77%) developed frequent falls. Using the severely disabling criteria, nine patients had frequent falls at baseline, and 31 patients subsequently developed frequent falls over time. Overall, a total of 40 patients (37.74%) developed frequent falls using the severely disabling criteria.
- Using the moderately disabling criteria, six patients had difficulty walking at baseline, and over time, 32 more patients developed difficulty walking. At their last follow-up, a total of 38 PD patients (35.85%) required assistance while walking. Using the severely disabling criteria, all patients could walk independently at baseline. However, at their last follow-up, five patients (4.72%) subsequently had difficulty walking (Figure 1C).
- At baseline, none of the patients showed signs of cognitive impairment, but two patients (1.89%) developed cognitive impairment over time when using the moderately disabling criteria; in contrast, when using the severely disabling criteria, none of the patients developed cognitive impairment.
- One patient had severe disability at baseline when the moderately disabling criteria were used. An additional 25 patients subsequently developed loss of autonomy over time, resulting in a total of 26 patients (24.53%) reporting loss of autonomy. Using the severely disabling criteria, all patients were independent at baseline. At their last follow-up, 12 patients (11.32%) had an H&Y score of 5, which indicates requiring a wheelchair or being bedridden (Figure 1D).
- Sex differences
- There were significant differences between sex and the development of dysarthria and dysphagia over time, with more males developing these symptoms than females. There were no significant differences found for the other milestones (Supplementary Table 4 in the online-only Data Supplement).
- Mortality
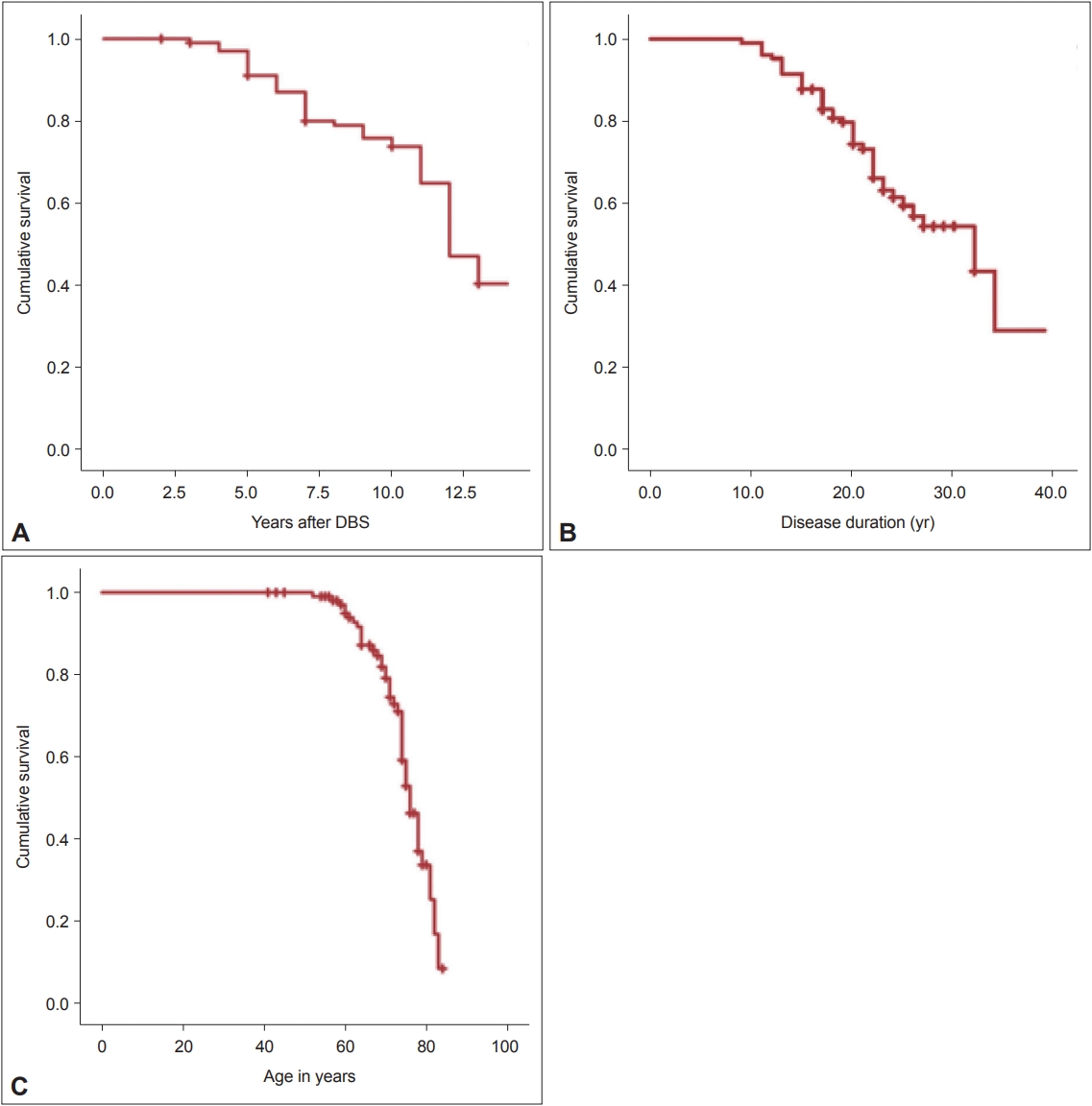
- Forty-two patients (39.62%) died after their last follow-up, but more than 75% of the patients survived to at least 10 years of follow-up after DBS surgery (Figure 2A). Half of the patients also reached a disease duration of 30 years and were still alive at the age of 75 (Figure 2C). Of the 42 reported deaths, the worsening of symptoms and pneumonia were reported as the most common causes of death in our patients (Table 4).
RESULTS
Hallucinations
Dysarthria
Dysphagia
Frequent falls
Difficulty walking
Cognitive impairment
Loss of autonomy
- In our study, we analyzed a prospectively collected dataset of 106 PD patients who underwent bilateral STN-DBS from 2005 to 2009. After the surgery, all the patients had significant improvement in their motor disability, with a concomitant decrease in the use of dopaminergic medications. However, in the subsequent follow-ups, the patients’ motor status slowly deteriorated. Medication dose was also slowly increased to compensate for the worsening status of the patients. However, even with medication adjustments, the natural course of the disease occurs, affecting the QoL of patients. Most of the patients developed some of the major clinical milestones, such as hallucinations, dysarthria, dysphagia, frequent falls, difficulty walking, cognitive impairment, and loss of autonomy, which also contributed to the functional capacity of the patients.
- After a postoperative follow-up of 10 years or more, 64 of the 106 patients were still alive, and all had active DBS electrodes. DBS was deemed to still be efficacious for their motor symptoms but not for their axial and nonmotor symptoms, which are usually poorly responsive or non-levodopa responsive. These symptoms often lead to impairments in the patients’ activities of daily living and may result in functional dependency for some. Our results are comparable with those of previous reports showing that patients will progressively develop disabling non-levodopa responsive symptoms even after undergoing DBS [5,11].
- In a previous report, the authors used very stringent criteria to identify the presence of key clinical milestones in patients [5]. Since the importance of the patients’ QoL has been emphasized several times in this study, we believe that using more lenient criteria would better reflect the impact of these milestones on the patients and their caregivers. In this study, two sets of criteria were chosen with two different sets of cutoff values. The first set is the severely disabling criteria, which are more stringent and use the highest scores possible for both the UPDRS and H&Y scales. The second set is the moderately disabling criteria, which are more lenient than the first set of criteria. As expected, more patients developed the milestones using the moderately disabling criteria than when using the severely disabling criteria, which was also shown as a clear separation between the KM curves of the two sets of data (Figure 1). Our results emphasized that using more lenient criteria for identifying key clinical milestones demonstrates a better picture of patients’ QoL. Even if the symptoms are not severe, they can still affect a patient’s functional capacity and can be burdensome for both patients and their caregivers.
- Comparing our results with those from a previously published study [5], the incidence was lower for all clinical milestones: hallucinations (9.43% vs. 60.87%), dysarthria (36.79% vs. 52.17%), dysphagia (15.09% vs. 34.78%), difficulty walking (35.85% vs. 52.17%), cognitive impairment (1.89% vs. 60.87%) and loss of autonomy (24.53% vs. 52.17%). A possible reason for these differences is that their sample population only included 23 patients. Another reason is that we used different criteria for each milestone. However, both studies emphasized that the presence of these milestones greatly affects the QoL of patients.
- In this study, less than 10% of the patients developed hallucinations over the observation period, which is inconsistent with previous reports wherein a higher percentage of PD patients developed hallucinations and psychosis whether they underwent DBS [5] or not [4,12]. A possible explanation for this is that we only included patients who had persistent hallucinations and excluded those who had transitory hallucinations. We also did not exclude patients taking antipsychotic medications, which may have addressed their hallucinations.
- For cognitive impairment, only two patients (1.89%) developed the symptom using the moderately disabling criteria, and none developed it using the severely disabling criteria. Our result goes against those of previous reports wherein a high percentage of medically treated [4,12,13] or DBS-treated [1,5,14] PD patients developed dementia over time. Neuropsychiatric assessments, scales and interviews would aid in accurately detecting cognitive impairment in these patients. Item 1 of the UPDRS (mentation), the K-MMSE and other neuropsychological battery tests were performed preoperatively to assess a patient’s overall cognitive function. Postoperatively, we did not perform full neuropsychological tests for all patients and only performed the UPDRS item 1, the K-MMSE and interviews. We opted to adhere to the UPDRS mentation score to avoid heterogeneity with the results. However, we report that this is one limitation of the study, since Item 1 of the UPDRS is not specific and the data gathered may be underrepresented.
- Our study showed that none of the patients developed severe dysphagia or cognitive impairment (UPDRS items 1 and 7, score of 4) over time, which is not consistent with previous reports [5,11]. We think that our data may be underrepresented because some of the patients may have died prior to developing the milestones. We can also say that those who did not develop severe dysphagia and cognitive impairment survived longer than those who did develop these symptoms.
- Our findings are inconsistent with previous reports highlighting that clinical milestones will emerge after a PD diagnosis, either in sequence [12] or not [5], and before death; seven out of the 42 patients who died in our study did not develop a single milestone and those who did develop a milestone did not develop all the milestones. Three of these patients had less than 6 years of follow-up, and we can assume that these patients did not have the chance to develop any milestones. On the other hand, our results support the concept of PD as a complex, multifactorial disease with clinical variability per individual [15]; hence, we do not expect all PD patients to develop the same symptoms. However, if clinical milestones are present, we can still use them as determinants of a patient’s QoL.
- Data from the current study showed a lower mortality compared to previously reported data [16-19]. This may be explained by several factors. First, there is an increasing life expectancy because of advancements in the field of medicine, with better treatments being readily accessible, leading to longer disease duration. Second, the overall mortality rate in South Korea is lower than that in other countries. Third, there might be selection bias in our study population since not all PD patients were eligible for DBS treatment. Fourth, the demographic data of our patients were different from those of patients in a previous study [19], wherein there were more women and a lower mean age at surgery. We can assume that our data on mortality might not be representative of the entire PD patient population.
- This study has several limitations. First, 17 of the 106 PD patients (16.0%) were lost to follow-up; therefore, some of the valuable clinical data were also lost. Second, we did not report on the other medications, such as anti-psychotics, used by the patients, which may affect the appearance of some milestones. Third, the 13-year follow-up period was chosen arbitrarily. This was the longest follow-up available during data collection because DBS was only started in our center in 2005. A previously published study had 15 years of follow-up, but it only had a small number of patients compared to ours. Fourth, the correlation between the development of the clinical milestones and the QoL of the patients would have emphasized the importance of the nonmotor and axial symptoms; however, this was not evaluated in this study. Last, the results cannot be directly applied to the general population of PD patients; there might be a possible selection bias since there was no control group with which to compare the results.
- In conclusion, our findings suggest that STN-DBS has a limited effect on axial and nonmotor symptoms of PD in contrast to the effect on motor symptoms. These symptoms may serve as clinical milestones that can convey the status of PD patients and its impact on the patients and their caregivers. Therefore, advanced PD patients, even those treated with bilateral STN-DBS, will still require assistance and cannot live independently in the long run.
DISCUSSION
Supplementary Materials
Supplementary Table 1
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None
-
Author Contributions
Conceptualization: Jed Noel A. Ong, Beomseok Jeon. Data curation: Jed Noel A. Ong, Beomseok Jeon. Formal analysis: Jed Noel A. Ong, Beomseok Jeon. Investigation: Jed Noel A. Ong, Beomseok Jeon. Methodology: Jed Noel A. Ong, Jung Hwan Shin, Han-Joon Kim, Beomseok Jeon. Project administration: Beomseok Jeon. Resources: Jed Noel A. Ong, Beomseok Jeon. Software: Jed Noel A. Ong, Jung Hwan Shin. Supervision: Han-Joon Kim, Sun Ha Paek, Beomseok Jeon. Validation: Han-Joon Kim, Beomseok Jeon. Visualization: Jed Noel A. Ong, Beomseok Jeon. Writing—original draft: Jed Noel A. Ong. Writing—review & editing: all authors.
Notes
- We are grateful to all the patients for their participation in this study. We also want to thank the staff of our Movement Disorder Center at Seoul National University Hospital and all other personnel involved in this study for their contributions.
Acknowledgments

|
Disabling criteria (n = 106) |
||
|---|---|---|
| Moderately | Severely | |
| Developed at least 1 milestone | 81 (76.41) | 48 (45.28) |
| Clinical milestones | ||
| Hallucinations | 10 (9.43) | 3 (2.83) |
| Dysarthria* | 39 (36.79) | 13 (12.26) |
| Dysphagia* | 16 (15.09) | 0 (0) |
| Frequent falls | 57 (53.77) | 40 (37.74) |
| Difficulty walking | 38 (35.85) | 5 (4.72) |
| Cognitive impairment | 2 (1.89) | 0 (0) |
| Loss of autonomy | 26 (24.53) | 12 (11.32) |
Values are presented as number of patients and percentage (%). Using the moderately disabling criteria, 81 of the 106 patients (76.41%) developed at least one clinical milestone after deep brain stimulation of the subthalamic nucleus (STN-DBS), while 48 patients (45.28%) developed a milestone using the severely disabling criteria. In both criteria, the most frequent clinical milestone reported was frequent falls.
* significant difference between sexes.
| Patient outcome | Result |
|---|---|
| Survival | 64 (60.38) |
| Death | 42 (39.62) |
| Worsening of symptoms | 14 |
| Pneumonia | 14 |
| Sepsis | 2 |
| Unknown cause | 7 |
| Other causes | 5 |
- 1. Rizzone MG, Fasano A, Daniele A, Zibetti M, Merola A, Rizzi L, et al. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: from the advanced phase towards the late stage of the disease? Parkinsonism Relat Disord 2014;20:376–381.ArticlePubMed
- 2. Romito LM, Contarino MF, Vanacore N, Bentivoglio AR, Scerrati M, Albanese A. Replacement of dopaminergic medication with subthalamic nucleus stimulation in Parkinson’s disease: long-term observation. Mov Disord 2009;24:555–561.Article
- 3. Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol 2011;68:1550–1556.ArticlePubMed
- 4. Hely MA, Morris JG, Reid WG, Trafficante R. Sydney multicenter study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 2005;20:190–199.ArticlePubMed
- 5. Constantinescu R, Eriksson B, Jansson Y, Johnels B, Holmberg B, Gudmundsdottir T, et al. Key clinical milestones 15 years and onwards after DBS-STN surgery—a retrospective analysis of patients that underwent surgery between 1993 and 2001. Clin Neurol Neurosurg 2017;154:43–48.ArticlePubMed
- 6. Kim R, Yoo D, Jung YJ, Lee WW, Ehm G, Yun JY, et al. Determinants of functional independence or its loss following subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg 2019;97:106–112.ArticlePubMed
- 7. Kim HJ, Jeon BS, Paek SH, Lee JY, Kim HJ, Kim CK, et al. Bilateral subthalamic deep brain stimulation in Parkinson disease patients with severe tremor. Neurosurgery 2010;67:626–632.discussion 632. ArticlePubMed
- 8. Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord 20080;23:1428–1434.Article
- 9. Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000;69:308–312.ArticlePubMedPMC
- 10. Opara JA, Brola W, Leonardi M, Błaszczyk B. Quality of life in Parkinson’s disease. J Med Life 2012;5:375–381.PubMedPMC
- 11. Merola A, Zibetti M, Angrisano S, Rizzi L, Ricchi V, Artusi CA, et al. Parkinson’s disease progression at 30 years: a study of subthalamic deep brain-stimulated patients. Brain 2011;134(Pt 7):2074–2084.ArticlePubMed
- 12. Bjornestad A, Pedersen KF, Tysnes OB, Alves G. Clinical milestones in Parkinson’s disease: a 7-year population-based incident cohort study. Parkinsonism Relat Disord 2017;42:28–33.ArticlePubMed
- 13. Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology 2008;70:1017–1022.ArticlePubMed
- 14. Kim HJ, Jeon BS, Paek SH, Lee KM, Kim JY, Lee JY, et al. Long-term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson’s disease. J Neurol 2014;261:1090–1096.ArticlePubMed
- 15. Campbell MC, Myers PS, Weigand AJ, Foster ER, Cairns NJ, Jackson JJ, et al. Parkinson disease clinical subtypes: key features & clinical milestones. Ann Clin Transl Neurol 2020;7:1272–1283.ArticlePubMedPMC
- 16. GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:939–953.PubMedPMC
- 17. Centers for Disease Control and Prevention. QuickStats: age-adjusted death rates* for Parkinson disease† among adults aged ≥65 years - National Vital Statistics System, United States, 1999-2017. MMWR Morb Mortal Wkly Rep 2019;68:773.ArticlePubMed
- 18. Bäckström D, Granåsen G, Domellöf ME, Linder J, Jakobson Mo S, Riklund K, et al. Early predictors of mortality in parkinsonism and Parkinson disease: a population-based study. Neurology 2018;91:e2045–e2056.ArticlePubMedPMC
- 19. Hitti FL, Ramayya AG, McShane BJ, Yang AI, Vaughan KA, Baltuch GH. Long-term outcomes following deep brain stimulation for Parkinson’s disease. J Neurosurg 2019;132:205–210.Article
REFERENCES
Figure & Data
References
Citations

- Unveiling the Impact of Outpatient Physiotherapy on Specific Motor Symptoms in Parkinson’s Disease: A Prospective Cohort Study
Yuta Terasawa, Koki Ikuno, Shintaro Fujii, Yuki Nishi, Emi Tanizawa, Sachio Nabeshima, Yohei Okada
Brain & Neurorehabilitation.2023;[Epub] CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite