Caregiver Burden of Patients With Huntington’s Disease in South Korea
Article information
Abstract
Objective
This is the first prospective cohort study of Huntington’s disease (HD) in Korea. This study aimed to investigate the caregiver burden in relation to the characteristics of patients and caregivers.
Methods
From August 2020 to February 2022, we enrolled patients with HD from 13 university hospitals in Korea. We used the 12-item Zarit Burden Interview (ZBI-12) to evaluate the caregiver burden. We evaluated the clinical associations of the ZBI-12 scores by linear regression analysis and investigated the differences between the low- and high-burden groups.
Results
Sixty-five patients with HD and 45 caregivers were enrolled in this cohort study. The average age at onset of motor symptoms was 49.3 ± 12.3 years, with an average cytosine-adenine-guanine (CAG)n of 42.9 ± 4.0 (38–65). The median ZBI-12 score among our caregivers was 17.6 ± 14.2. A higher caregiver burden was associated with a more severe Shoulson–Fahn stage (p = 0.038) of the patients. A higher ZBI-12 score was also associated with lower independence scale (B = -0.154, p = 0.006) and functional capacity (B = -1.082, p = 0.002) scores of patients. The caregiving duration was longer in the high- than in the low-burden group. Caregivers’ demographics, blood relation, and marital and social status did not affect the burden significantly.
Conclusion
HD patients’ neurological status exerts an enormous impact on the caregiver burden regardless of the demographic or social status of the caregiver. This study emphasizes the need to establish an optimal support system for families dealing with HD in Korea. A future longitudinal analysis could help us understand how disease progression aggravates the caregiver burden throughout the entire disease course.
INTRODUCTION
Huntington’s disease (HD) is a rare disease with an annual incidence of 0.29 per 100,000 people and a 10-year prevalence of 2.2 per 100,000 people reported by a recent Korean study [1]. Although it is rare, HD has a great medical impact because the disease shows full penetrance among family members and anticipation leading to earlier disease onset in younger generations [2,3]. By analyzing the 10-year diagnostic records and medical expenses of HD in Korea recorded in the Health Insurance and Review Assessment Service database, we previously reported that almost half of the patients did not visit the hospital regularly after diagnosis, and eventually most of them were lost to follow-up [1].
It is necessary to provide comprehensive care after a diagnosis of HD to have a public health system that supports patients, gene carriers, and caregivers. In Europe and North America, large-scale HD cohorts have been established to investigate the underlying mechanism of clinical progression and the appearance of complex neurological symptoms [4-6]. Active clinical trials based on the cohort are underway to develop neuroprotective therapeutics and advanced gene therapy [7]. However, poor recognition of the disease and lack of social infrastructure to support families with HD are unresolved issues in Korea. Therefore, it is necessary to establish a Korean HD cohort to promote therapeutic development and find ways to maintain patients’ quality of life.
Family members often take care of patients in a complicated situation because they might be potential gene carriers themselves. Therefore, caregivers might endure the disease burden while taking a crucial role in patient care. Caregiver distress is shown to be significantly high in HD compared to other neurological conditions [8]. Several studies have revealed the caregiver burden of HD in other countries (Supplementary Table 1 in the online-only Data Supplement). The caregiver burden of HD seems to be affected by patients’ motor disturbance, dependency, and functional capacity as well as by the discrepancy between reality and their expectations of life and patients’ nonmotor disabilities, such as irritability, obsessive-compulsive behaviors, depression, anosognosia, and cognitive dysfunction [9-15]. On the other hand, the burden is relatively low for patients with the apathetic predominant type [9]. In addition, regional and ethnic differences should be considered since the caregiver burden is related to social and cultural factors. However, most studies have been conducted in Western countries thus far, and it is unknown what factors affect the burden of caregivers of HD patients in Korea. Therefore, this study aimed to investigate the caregiver burden and associated factors of HD patients by analyzing the baseline data of a prospective Korean HD cohort.
MATERIALS & METHODS
Study participants
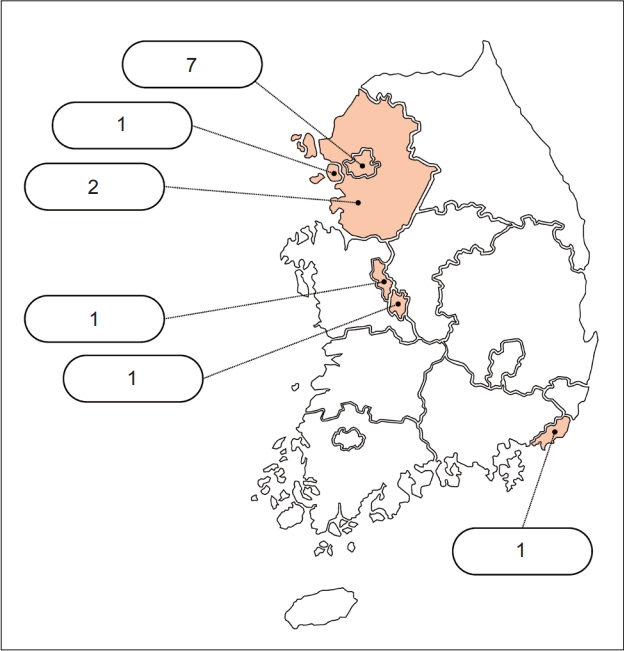
This is the first study to establish a multicenter prospective Korean HD cohort. During the enrollment period of 19 months between August 2020 and February 2022, patients with manifest HD, asymptomatic gene mutation carriers who did not show clinical symptoms (premanifest HD), and nongenetic carriers in the patients’ families were enrolled in the prospective cohort from 13 referral hospitals in Korea (Figure 1).
Clinical assessment
We collected information on characteristics such as age, sex, age at symptom onset, disease duration, and cytosine-adenineguanine (CAG) repeat number. Clinical assessments included the Unified Huntington’s Disease Rating Scale (UHDRS) Parts I and IV, Montreal Cognitive Assessment (MoCA), Mini-Mental State Examination (MMSE), Beck Depression Inventory (BDI), Geriatric Depression Scale (GDepS), and Neuropsychiatric Inventory (NPI). We did not evaluate UHDRS Part II or III. We assessed patients’ functional stage with Shoulson–Fahn (SF) staging.
Assessment of caregivers’ burden and characteristics
The caregiver burden was evaluated using the 12-item Zarit Burden Interview (ZBI-12) [16,17]. The ZBI is a widely used, selfreport instrument that measures the caregiver burden and was originally developed as a 29-item questionnaire [18]. Later, various short versions of the ZBI were developed because the length of the original ZBI was an obstacle to its use in clinical practice and research. We also collected information about the age, sex, education level, marital status, blood relation, and caregiving duration of the caregivers.
Statistical analysis
Participants’ and caregivers’ characteristics are presented as the means ± standard deviations (SDs) for continuous variables and frequencies and percentages for categorical variables. Differences among the groups were evaluated using the chi-square test for categorical variables and Student’s t test or Mann‒Whitney U test for continuous variables, depending on the normality of the data. Any correlation between the variables was investigated by Pearson correlation analysis. The characteristics of patients and caregivers were compared between the low- and highburden groups dichotomized by the median value (< 26 vs. ≥ 26) of the ZBI-12 scores. To identify factors that predicted the caregiver burden, a linear regression analysis was performed with the ZBI-12 score as a dependent variable. Multivariable regression models were built adjusting for onset age, disease duration, and CAG repeat number. All statistical analyses were performed using IBM SPSS 25.0 software (IBM Corp., Armonk, NY, USA) with significance set at p < 0.05.
This study was approved by the Institutional Review Board (IRB) of each participating center (representative protocol number of the IRB of the Seoul Metropolitan Government-Seoul National University Boramae Medical Center: 20-2020-15), and written informed consent was obtained from all participating individuals. All procedures performed in studies involving human participants were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
RESULTS
Baseline characteristics of the subjects
A total of 74 patients, including 65 patients with HD, six premanifest HD patients, and three family members without gene mutations, were enrolled in this cohort. Among them, data from the 65 HD patients were included in this study (Table 1). The mean age of the patients was 56.3 ± 12.5 years, and 22 (33.8%) were males. The average onset age of motor symptoms was 49.3 ± 12.3 years; 4 (6.1%) patients developed symptoms before 30 years of age, while the onset age was 60 years old or later among 12 (18.5%) patients. The average CAG repeat length was 42.9 ± 4.0 (range 38–65). An inverse correlation between (CAG)n and onset age was demonstrated in this cohort (R = -0.546, p < 0.001; Supplementary Figure 1 in the online-only Data Supplement). The overall MoCA and MMSE cognition scores of HD patients were 15.4 ± 8.3 and 20.7 ± 6.8, respectively. The average BDI score of HD patients was 16.3 ± 13.6.
Patient and caregiver characteristics and burden
A total of 45 caregivers participated in this cohort. The caregivers’ characteristics and ZBI-12 scores are summarized in Table 2. The average age of the caregivers was 54.3 ± 13.6 years, and 53.3% were male. Thirty-five (77.8%) of the caregivers had 10 years or more of schooling. Twenty-five (55.5%) were the nonblood relatives of patients, and 51.1% of caregivers were spouses. The average duration of caregiving was 4.9 ± 3.8 years. Two caregivers did not report their caregiving duration. The average ZBI-12 score was 17.6 ± 14.2.
In the comparison of characteristics of patients and caregivers between caregiver groups with low and high ZBI-12 scores (Table 3), the UHDRS Part IV-C (functional capacity) score was significantly lower (low burden vs. high burden = 8.0 ± 3.8 vs. 5.4 ± 4.3, p = 0.039), and the caregiver duration was longer (low burden vs. high burden = 3.2 ± 6.4 vs. 6.4 ± 4.2, p = 0.003) in the high burden group than in the low burden group.
We further compared characteristics between the patient groups following SF staging (Supplementary Table 2 in the online-only Data Supplement). The ZBI-12 scores of caregivers were also higher for more severe SF stages of the patients.
Factors associated with the caregiver burden of HD
The association between the ZBI-12 and the characteristics of HD patients was evaluated by linear regression analysis (Figure 2, Table 4). Among the baseline characteristics of the patients, UHDRS part IV-B (independence) (B = -0.154, p = 0.006) and IV-C (functional capacity) (B = -1.082, p = 0.002) scores were significantly associated with the caregivers’ ZBI-12 scores (Figure 2A, B). Other patient characteristics, such as age, cognition (MoCA), depression (BDI), and UHDRS motor scale score, did not significantly affect the ZBI-12 score. On the other hand, caregiver burden increased with lower MMSE scores (B = -0.367, p = 0.075).
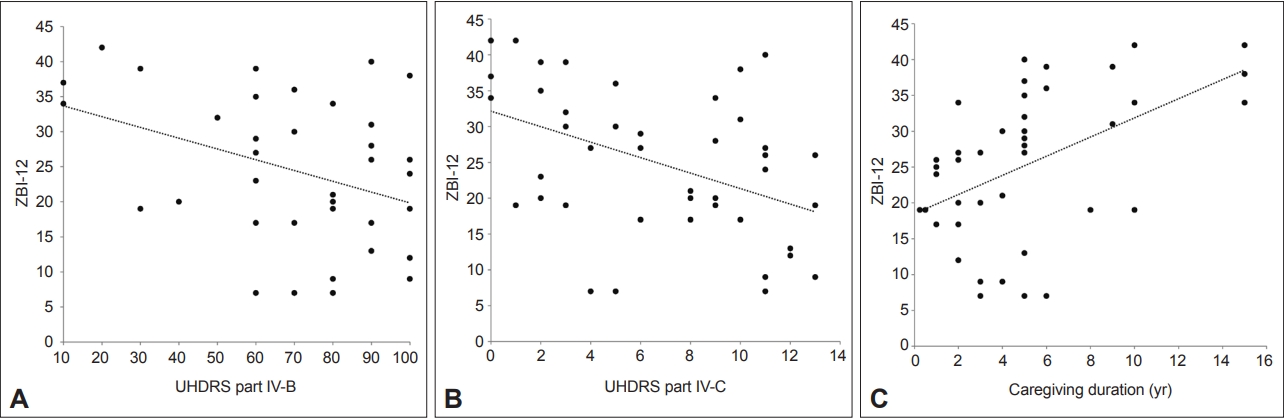
Factors associated with the caregiver burden of HD patients in the regression analysis. A: UHDRS part IV-B (independence scale) and ZBI-12 (B = -0.154, p = 0.006, 95% CI [-0.261, -0.047]). B: UHDRS part IV-C (functional capacity) and ZBI-12 (B = -1.082, p = 0.002, 95% CI [-1.751, -0.414]). C: Caregiving duration and ZBI-12 (B = 1.339, p = 0.001, 95% CI [0.609, 2.069]). HD, Huntington’s disease; UHDRS, Unified Huntington’s Disease Rating Scale; ZBI-12, 12-item Zarit Burden Interview; CI, confidence interval.

Results of the linear regression analysis of the patient characteristics that affected the ZBI-12 score
To evaluate the influence of the functional capacity of HD patients on each characteristic of the ZBI-12 score, we conducted multivariable linear regression analysis by entering each characteristic and adjusting for onset age, disease duration, and CAG repeat number. Independence and functional capacity were analyzed by dividing them into multivariable linear analyses 1 and 2, respectively, due to multicollinearity (Table 4 and 5). In the multivariable analyses, independence (UHDRS part IV-B) and functional capacity (UHDRS part IV-C) were found to have a significant effect on the caregiver burden (Table 4).

Results of the linear regression analysis of the caregiver characteristics that affected the ZBI-12 score
Regarding the caregivers’ characteristics, a longer caregiving duration predicted a higher ZBI-12 score in the univariable analysis (Figure 2C). However, the caregiving duration was not significant in the multivariable analysis adjusting for onset age, disease duration, and CAG repeat number. Other caregiver characteristics did not show any significant relationship with the ZBI-12 score (Table 5).
DISCUSSION
This study is the first report on the burden of caregivers of HD patients in an Asian country. The results of this study showed potential differences in the caregiver burden between Eastern and Western societies.
The average ZBI-12 score of the caregivers in this study was 17.6 ± 14.2, which was higher than the cutoff value of 12 points, indicating high caregiver stress, and was above the average score (15.1 ± 10.0) reported among caregivers of patients with common dementia disorders [16]. No study has evaluated the caregiver burden in HD using the ZBI-12, but two studies have used the full version of the 22-item ZBI (Supplementary Table 1 in the online-only Data Supplement). One study showed an average ZBI score of 33.8 [9], while the other study showed ZBI scores of 38.4 and 22 for caregivers of HD patients with and without anosognosia, respectively [15]. Some studies have evaluated the caregiver burden with the Caregiver Burden Inventory or Modified Caregiver Strain Index. By carefully reviewing the literature and our data, we found that the caregiver burden of HD patients in Korea was high, which was consistent with previous reports in other countries [9-13,15].
The present study provides useful insight into the factors associated with the caregiver burden in Korea. Previous studies have shown that advanced disease and low functional capacity were associated with the caregiver burden [9,10,12]. It makes sense that as patients’ function declines, their dependency on caregivers increases. However, functional capacity is not solely determined by chorea or other motor symptoms in HD. Motor symptom severity, assessed on the motor part of the UHDRS, has shown controversial effects on the caregiver burden. One study reported no association as was found in this study [13], while another study showed a significant effect of the severity of motor disturbance [14].
In addition to motor disability, nonmotor symptoms can be important determinants of the caregiver burden in HD. The caregiver burden increased with lower MMSE scores (B = -0.367, p = 0.075). Consistent with our findings, cognitive dysfunction in HD was demonstrated to be associated with the caregiver burden in previous studies, and similar associations were found in studies on common dementias [11,13,19,20]. For the comparison, we provided a summary table of the caregiver burden for common dementia [21-23] and Parkinson’s disease [24-27] in Korea (Supplementary Table 3 in the online-only Data Supplement). One study used the ZBI-12 to investigate caregiver intervention among community-based individuals with dementia [22]. In that study, the average caregiver burden score among the participants aged similar to the present cohort was 18.0 ± 6.1, which was in a similar range to our data. Other studies used other versions of the ZBI, showing that the caregiver burden of individuals with common dementia or Parkinson’s disease was approximately 3 to 5 times higher than that of normal elderly individuals [21,23-27]. Direct comparison of scores with the results of this study was impossible because the scales were different, so we estimated the percentage of the caregiver burden by calculating the mean burden score/maximum score × 100. The estimated percentage of caregivers in this study (36.6%) was comparable with those in other studies [23,25-27]. One study reported a lower percentage than that in this study (17.8%), which could be explained by a community-based design that included more patients with minor symptoms [21]. Another study showed a higher percentage (67.4%) than other studies, including this study [24]. The reason for the high score in that study was unknown.
Unlike a previous study in Western countries [14], depressive symptoms were not associated with the caregiver burden in this Korean cohort. The patients’ overall social performance and activities of daily living (ADLs) and the dependency on a caregiver seemed to be more important for the caregiver burden in Korea than impairments in specific symptom domains, such as motor, cognitive and psychiatric symptoms. Continuous caregiver management seems to be necessary in HD, as both motor and nonmotor (cognitive and psychiatric) symptoms of HD patients progress over time, complicating the patients’ ADLs.
We also evaluated whether the characteristics of caregivers were associated with the burden of caregiving. We found that caregiving duration was longer in the high-burden group than in the low-burden group. Moreover, a positive relationship between caregiving duration and burden was found in the univariable linear regression analysis. Only two studies have reported an association between caregiver characteristics and their burden of caregiving [12,13]. In those studies, younger caregiver age and being the sole caregiver were significantly associated with a higher burden. Our results imply that caregiving duration may be another predictor of the caregiver burden. To elucidate the relationship between caregiver characteristics and burden, further studies that include a larger number of caregivers and a more detailed collection of caregiver characteristics are needed.
This study was conducted using the baseline characteristics of the first prospective multicenter HD cohort in Korea. Because this is the first cohort, there are several limitations; the number of patients included in the analysis was relatively small because the prevalence of HD in Korea is lower than that in countries in Europe and America [1,28]. Additionally, no follow-up data have been analyzed thus far. However, cohort enrollment has been recently expanded to 32 referral hospitals from almost all geographical regions of South Korea. This extended ongoing cohort will be a foundation for the standardized care of Korean patients with HD and their caregivers and will provide a valuable platform for a high-quality longitudinal study that includes imaging and laboratory biomarkers and a potential source for clinical trials of disease-modifying therapy for HD in Korea in the future.
In conclusion, the caregiver burden of HD patients was mostly affected by the patients’ dependency and functional capacity. Any efforts to maintain patients’ independent daily activities would alleviate the burden of caregivers in families with HD. Further studies with detailed evaluations of socioeconomic and cultural factors are worth conducting to reveal culture-specific risk factors. Longitudinal analysis of the prospective cohort data will help to develop an appropriate support system for caregivers of HD patients throughout the entire disease course.
Supplementary Material
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.23134.
Supplementary Figure 1.
The relationship between CAG repat number and onset age of HD patients enrolled in this study. The age at onset correlated negatively with CAG repeat number (r = -0.546, p < 0.001 by Pearson correlation analysis). CAG, cytosine-adenine-guanine; HD, Huntington’s disease.
Supplementary Table 1.
Summary of studies on caregiver burden in HD
Supplementary Table 2.
Characteristics of HD patients according to the SF staging
Supplementary Table 3.
Summary of studies on caregiver burden in common dementias and PD using ZBI in Korea
SUPPLEMENTARY REFERENCES
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
This work was supported by the Seoul Metropolitan Government-Seoul National University Boramae Medical Center (SMG-SNU BMC) focused clinical research grant-in-aid (04-2022-0014).
Author contributions
Conceptualization: Jee-Young Lee, Eungseok Oh, Won Tae Yoon. Data curation: Chan Young Lee, Jee-Young Lee, Eungseok Oh, Chaewon Shin, Yun Su Hwang. Formal analysis: Chan Young Lee, Chaewon Shin. Funding acuiqition: Jee-Young Lee. Investigation: Jee-Young Lee, Chan Young Lee, Chaewon Shin, Eungseok Oh, Yun Su Hwang, Won Tae Yoon, Manho Kim, Hyun Sook Kim, Sun Ju Chung, Young Hee Sung, Jin Whan Cho, Jae-Hyeok Lee, Han-Joon Kim, Hee Jin Chang, Beomseok Jeon, Kyung Ah Woo, Kyum- Yil Kwon, Jangsup Moon, Young Eun Kim, Seong-Beom Koh. Methodology: Jee-Young Lee, Chan Young Lee, Chaewon Shin, Eungseok Oh. Project administration: Jee-Young Lee, Eungseok Oh. Resources: Jee-Young Lee, Chan Young Lee, Chaewon Shin, Eungseok Oh, Yun Su Hwang, Won Tae Yoon, Manho Kim, Hyun Sook Kim, Sun Ju Chung, Young Hee Sung, Jin Whan Cho, Jae-Hyeok Lee, Han-Joon Kim, Hee Jin Chang, Beomseok Jeon, Kyung Ah Woo, Kyum-Yil Kwon, Jangsup Moon, Young Eun Kim, Seong-Beom Koh. Supervision: Jee-Young Lee, Manho Kim, Eungseok Oh. Validation: Chan Young Lee, Chaewon Shin, Jee-Young Lee, Eungseok Oh. Visualization: Chan Young Lee, Chaewon Shin. Writing—original draft: Chan Young Lee, Chaewon Shin. Writing—review & editing: Jee-Young Lee, Chan Young Lee, Chaewon Shin, Eungseok Oh, Yun Su Hwang, Won Tae Yoon, Manho Kim, Hyun Sook Kim, Sun Ju Chung, Young Hee Sung, Jin Whan Cho, Jae-Hyeok Lee, Han-Joon Kim, Hee Jin Chang, Beomseok Jeon, Kyung Ah Woo, Kyum-Yil Kwon, Jangsup Moon, Young Eun Kim, Seong-Beom Koh.
Acknowledgements
We thank the patients and their families for their willingness to participate in this cohort study.