Alternating Hemiplegia of Childhood in a Person of Malay Ethnicity with Diffusion Tensor Imaging Abnormalities
Article information
Alternating hemiplegia of childhood (AHC) is a rare neurodevelopmental disorder with an incidence of one per million, characterized by paroxysmal alternating hemiplegia, eye movement abnormalities, dystonia, and epilepsy [1]. In 2012, mutations in the ATP1A3 gene encoding the Na+-K+-ATPase α3 subunit (originally discovered in rapid-onset dystonia-parkinsonism) were identified as the cause for AHC [1,2]. However, the diagnosis of AHC is still often delayed or missed. We report a case of AHC diagnosed in adulthood; to our knowledge, this is the first report in a person of Malay ethnicity. There are very few studies utilizing more advanced neuroimaging in AHC, and none have specifically used diffusion tensor imaging (DTI). DTI allows detection of loss of white matter tract (WMT) integrity even when conventional MRI appears normal [3]. Here, we documented novel DTI abnormalities in AHC.
A 24-year-old Malay woman with global developmental delay since infancy was referred with a seven-year history of recurrent painful paroxysmal left hemidystonia. The frequency of attacks ranged from 3–4 times daily to 2–3 times a month. Each attack usually lasted a few minutes and occasionally up to one hour. Triggers included excitement, bright lights, and crowded places. There was no association with sudden movements or prolonged exercise. Interictally, she could ambulate and perform activities independently. Family history was negative. Further probing revealed a history of paroxysmal hemiplegia, alternating from side to side, starting at age 13 months. Each hemiplegic episode lasted a few days and occurred 4–5 times a month with gradual resolution by age two years.
General examination revealed normal stature with no visual, hearing or bony abnormalities. There was apraxia of horizontal eye movements, severe dysarthria, and right torticollis (Supplementary Video 1 in the online-only Data Supplement). There was dystonic posturing in the fingers, which was worse on the left and more pronounced during writing. Repetitive hand movements were slow. There was no tremor, rigidity or dysmetria. Limb power was full with normal tendon and plantar reflexes. Gait was mildly ataxic. The Montreal Cognitive Assessment score was 4/30. Two episodes of painful hemidystonia lasting a few minutes were witnessed during consultation, with left-sided wrist flexion and ankle inversion.
Routine blood tests, brain MRI and electroencephalogram were normal. Analysis of the ATP1A3 gene confirmed the diagnosis of AHC with a previously reported mutation at NM_152296.4:c.2839G>A (rs398122887), resulting in amino acid substitution NP_689509:p.(Gly947Arg) [2]. This mutation was absent in her mother and two sisters; the patient’s father was unavailable for testing. Combination treatment with flunarizine, baclofen and clonazepam reduced the frequency, duration, and severity of the hemidystonic attacks. There was no response to topiramate, and sodium oxybate was unavailable.
The DTI dataset of the patient was obtained during the interictal state using single-shot echo-planar imaging and was compared to our database of 25 normal controls with a similar mean age (29.1 ± 7.1 years). The controls were used to build a normal distribution, and their values defined the ‘normal range’ of mean ± 1.96 standard deviations (equivalent to p = 0.05 on two-tailed testing). We then utilized the Tract-Based Spatial Statistics functionality of the FSL toolkit (FMRIB Analysis Group, Oxford, UK) to register all volumes to standard Montreal Neurological Institute space and to produce the overall “skeletonized” tract mask. From this, we extracted mean values based on regions of interest utilizing the International Consortium on Brain Imaging (ICBM-81) atlas as an anatomical guide [4]. Despite the left-sided dominance in clinical presentation, DTI abnormalities (Figure 1) were observed mainly in the ipsilateral (i.e., left-sided) WMTs, especially in the anterior limb and retrolenticular part of the internal capsule, anterior corona radiata, external capsule, cingulum, fornix, superior longitudinal and fronto-occipital fasciculus. Changes on the right were limited to the anterior limb of the internal capsule and cingulum. The mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) values were significantly higher in the patient than in the controls, with no difference in fractional anisotropy (FA) (Supplementary Table 1 in the onlineonly Data Supplement).
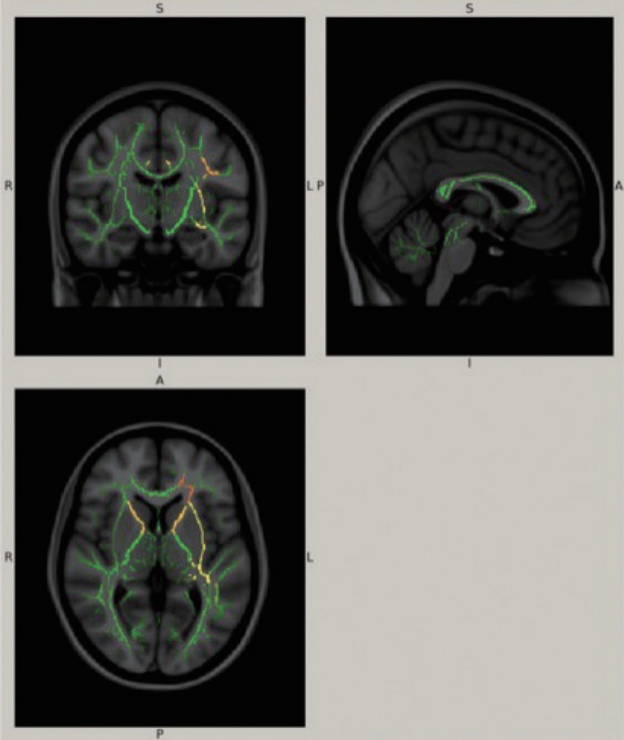
A standard brain template with composite of fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity values overlaid on each other illustrating the locations of significant differences between the patient and normal controls. Green represents the mean white matter skeleton of all the subjects. The yellow, orange and red areas denote the predefined regions-of-interest, where the alternating hemiplegia of childhood patient values differ significantly from those of normal controls. The magnitude of the difference increases from red to yellow.
Numerous genetic, immune-mediated, vascular, metabolic and traumatic conditions have been associated with paroxysmal dystonia; ATP1A3 mutations are a rare cause [5]. AHC is usually diagnosed in childhood, with symptoms typically appearing in the first 18 months of life [1,6,7]. The classic hemiplegic attacks usually decrease in frequency as the patient gets older [1], making the diagnosis a challenge in adulthood, at which time nonparoxysmal manifestations such as intellectual disability, ataxia or choreodystonia become the predominant features.
More than 34 ATP1A3 mutations have been reported in AHC, with most cases being sporadic [6,7]. The G947R mutation is the third most common mutation detected after D801N and E815K [6] and is associated with a milder phenotype, including later onset, less frequent plegic episodes and milder intellectual disability, but with a tendency for more frequent/longer dystonic attacks [6,7], as seen in this case. In preclinical studies, AHC-causing mutations resulted in reduced ATPase activity without affecting protein expression levels [2], and loss of proton transport correlated with disease severity [1].
Conventional brain MRI is usually normal, although progressive brain atrophy has been reported in severe AHC [1]. Brain perfusion, metabolism and spectroscopic abnormalities have been demonstrated in single case reports [1]. Increased MD, RD, and AD suggest loss of WMT integrity and possible neurodegeneration [3], which in our case of milder AHC phenotype is interesting, as it has been suggested that AHC is not an intrinsically progressive disease (with the exception of those harboring E815K mutations) [1,6,7]. There was no significant difference in FA between our patient and controls, possibly due to the small sample size. The marked asymmetry in WMT abnormalities is consistent with the asymmetrical clinical pattern, although why the changes in this case were more pronounced in the cerebral hemisphere ipsilateral to the hemidystonia is unclear. Abnormalities of both white matter and gray structures have been reported in disorders with ATP1A3 mutations [8], although notably no neuropathological examination of AHC cases has been reported to date. As we have only studied a single patient, further studies are needed to understand the nature and pathogenesis of WMT changes in AHC. DTI characterization of gray matter is challenging and relatively unexplored.
This first report of AHC in Malay ethnicity highlights the importance of obtaining a careful developmental history when approaching a case of paroxysmal dystonia presenting in adulthood and expands the ethno-epidemiology of ATP1A3 mutations.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.18063.
Supplementary Video Legends
Video 1. Segment 1 shows oculomotor apraxia, severe dysarthria and dystonia involving the neck and upper limbs. Repetitive hand movements were slow. There was no dysmetria. Gait was slow and mildly ataxic with inability to perform tandem gait. Segment 2 shows an episode of painful left-sided hemidystonia.
Supplementary Table 1
Differences in DTI values in ROIs that were significantly different between AHC patient and controls
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Acknowledgements
This work was supported by the Parkinson’s Disease and Movement Disorders Research Program, University of Malaya (PV035-2017); University of Malaya Research Grant (RP052B-17HTM); and NINDS R01NS058949 (AB).
A written consent was obtained from the patient’s legal guardian prior to the publication of this case report. We would like to acknowledge the patient and her family for their participation in this case report.
