Movement Disorders in Non-Wilsonian Hepatic Cirrhotic Patients: The Subgroup Analysis of Various Phenotypes and Associated Risk Factors
Article information
Abstract
Objective
The aim of this subgroup analysis was to identify the risk factors associated with the development of various movement disorder phenotypes.
Methods
Eighty-three non-Wilsonian cirrhotic patients with abnormal movements were allocated into the following groups: intention tremor, bradykinesia, Parkinsonism, and abnormal ocular movements. These movement types were considered the primary outcomes as there was a sufficient sample size. Researchers took into consideration the gender, etiologies of cirrhosis, cirrhosis-related complications, hepatic encephalopathy, medical illness, and some neurological deficits as potential factors associated with these movement disorders.
Results
The male gender (p = 0.002) and alcoholic cirrhosis (p = 0.005) were significant factors for the prevalence of intention tremors. In bradykinesia, hepatic encephalopathy was highly statistically significant (p < 0.001), and females more commonly developed bradykinesia (p = 0.04). The Parkinsonism features in this study were confounded by hyperlipidemia (p = 0.04) and motor or sensory deficits (p = 0.02). Jerky pursuits and a horizontal nystagmus were detected. Jerky pursuits were significantly related to hepatic encephalopathy (p = 0.003) and bradykinesia, but there were no factors associated with the prevalence of nystagmus other than an intention tremor.
Conclusions
The association of alcoholic cirrhosis with the development of intention tremor indicates that the persistent cerebellar malfunction in cirrhotic patients is due to alcohol toxicity. The slowness of finger tapping and jerky pursuit eye movements are significantly associated with hepatic encephalopathy. Thus, further studies are needed to evaluate the diagnostic value of these two signs for an early detection of mild hepatic encephalopathy.
Since the clinical and pathological studies of Victor, Adams, and Cole in 1965, movement disorders in acquired hepatic cirrhotic patients have been acknowledged. The term “chronic acquired hepatocerebral degeneration (CAHD)” refers to neurological deficits that include various abnormalities such as ataxia, dysarthria, and cognitive deterioration [1]. Although the asterixis or negative myoclonus resulting from hyperammonemia is episodic and reversible in hepatic encephalopathy, Parkinsonism and other movement disorders in CAHD are permanent. The pathogenesis of CAHD is still unknown. Although some researchers have proposed that CAHD might be associated with an extensive portosystemic shunt, there are some reports of patients presenting with neurological deficits who do not have hepatic signs [2]. A pathological case reported in 1970 revealed degenerative processes that involved the basal ganglia, cerebral cortex, white matter, brainstem, and cerebellum [3]. This finding explains various neurological manifestations and movement disorders found in patients with CAHD.
Other abnormal movements related to CAHD include hemiballism [4], extraocular muscle dystonia [5], orofacial dyskinesia [6], and tremors that can be intentional, postural, or rest tremors.1 However, tremors, ataxia and other movement disorders in chronic liver disease are also influenced by many factors such as alcohol [7], medications [4], and metabolic imbalances [8]. Furthermore, there is one case report of recurrent neurological deficits and Parkinsonism in a cirrhotic patient following liver transplantation [9]. All these factors bring into question other etiologies that may contribute to the prevalence of various movement disorder phenotypes. If these etiologies are reversible, patients can benefit from early diagnosis, treatment, and prevention. This event is especially true for some conditions that are associated with hepatic encephalopathy. This report aims to reveal the details of each of the movement disorder phenotypes and to determine the possible related factors.
MATERIALS & METHODS
Study population
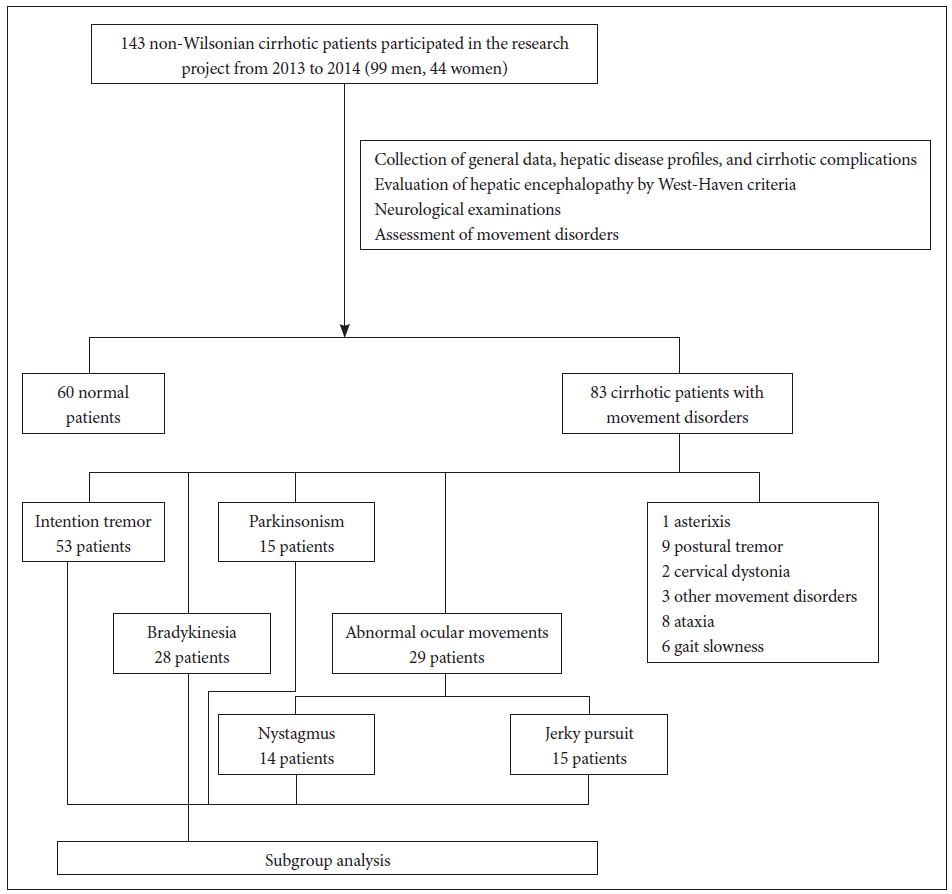
The Ethical Committee of the Faculty of Medicine, Srinakharinwirot University approved the research proposal, the data recording, consent forms, and the patients’ information. This study included 143 volunteers (99 men, 44 women) who were diagnosed with non-Wilsonian hepatic cirrhosis using the inclusion criteria as classified in the first report. Two gastroenterologists collected personal data, the patients’ medical histories, the etiologies of cirrhosis, and the information on medical comorbidities. Upper abdominal ultrasonography or computed tomography (CT) scans of the abdomen were used to confirm hepatic cirrhosis. Liver biopsies were investigated in cases with suspicious hepatic malignancies. A neurologist performed the neurological and movement disorder examinations. All subjects had normal consciousness and did not have lifethreatening conditions. Eighty-three volunteers presenting with various movement disorders were categorized and preselected for a subgroup analysis as illustrated in Figure 1.
Outcomes
Movement disorders and neurological deficits
Asterixis, postural tremor, intention tremor, Parkinsonism, bradykinesia, dystonia, abnormal ocular movements, and an unsteady gait were all movement disorder phenotypes detected in this study. Only the group of patients with intention tremor, bradykinesia, Parkinsonism, and abnormal ocular movements had a sample size large enough (n > 10) to be included in the subgroup analysis. The intention tremor was characterized as a mild action tremor. It presented while performing the finger-to-nose test. The tremor was evident when the index finger approached the target without pointing beyond the target. Patients with bradykinesia in the subgroup analysis were determined to be those with a certain degree of sluggish manual dexterity (finger tapping, hand closing and opening, and rapid alternation) and/or leg agility without the presence of cogwheel rigidity. The slowness of finger tapping had a delayed onset without a decrease in the tapping width. Due to the lack of a measuring instrument to identify the rate or frequency of hand movements, the researcher assessed the slowness of finger tapping in the patients by comparing them with the finger tapping in healthy subjects. To diagnose Parkinsonism, patients were required to present with at least two of the following signs: resting tremor, rigidity, bradykinesia and postural instability. We also used the Unified Parkinson’s Disease Rating Scale (UPDRS), part three, to estimate the severity of Parkinsonism. Abnormal ocular movements were divided into a group with nystagmus and a group with jerky pursuit. Hyposmia is the neurological deficit that theoretically yields a significant association with idiopathic Parkinson’s disease. To screen for hyposmia in this study, we used two tubes containing coffee and soap as objects for the smelling test. The nostrils of the volunteers were examined, and patients were asked to close their eyes and describe the odor. There were some cases with impaired smelling function, and the researchers analyzed its relationship to Parkinsonism.
Independent variables and confounders
General data, cirrhosis, and other medical illnesses
Gender was one of the independent variables. The age range of the subjects was 37–70 years of age. Researchers divided the subjects into three groups of 37–49, 50–59, and 60–70 years of age. Hepatitis B virus, hepatitis C virus, alcoholism, non-alcoholic steatohepatitis (NASH), cryptogenic, and combined causes of cirrhosis were considered to be potentially independent variables. Patients who had at least two risk factors for the development of cirrhosis were considered to have combined causes of cirrhosis. For example, one patient had hepatitis B and hepatitis C, and another patient had hepatitis C and alcoholic cirrhosis. Non-neurological complications such as portal hypertension and hepatocellular carcinoma were considered as variables that could be associated with the prevalence of movement disorders. The Child-Turcotte-Pugh (CTP) score of each patient was accessed and recorded. Other medical illnesses that may play a role in the presence of neurological deficits and/or movement disorders such as diabetes mellitus (DM), hypertension, hyperlipidemia, a prior history of cerebrovascular disease, impairment of renal function, and coronary artery disease were regarded as confounding factors. The history of alcohol consumption was one of the confounders that could result in some movement disorders. This variable was used to describe volunteers who continued to drink a different volume of alcohol and those who had stopped drinking.
Neurological and neuropsychiatric examinations
There were three clinical manifestations that were considered potential factors for the prevalence of movement disorders: motor and sensory deficits, motor or sensory deficits, and hepatic encephalopathy. Decreased motor strength, impairments of pain sensation and/or proprioception may be relevant to bradykinesia and Parkinsonism. Thus, investigators classified these variables as confounders. The neurological deficits were divided into two groups: cases with both motor and sensory deficits and cases with either motor or sensory deficits. Cirrhotic patients in the motor and sensory deficits group presented clinically with both motor weakness and impaired sensory modalities. Patients in the motor or sensory deficits group included volunteers who had a motor weakness, decreased pain sensation or impaired proprioception. The definition of hepatic encephalopathy was described in the Working Party at the 11th World Congress of Gastroenterology [10]. Hepatic encephalopathy in this study was classified as a type C encephalopathy associated with cirrhosis, portal hypertension and/or portal-systemic shunting. Researchers classified the severity of the encephalopathy using the West Haven criteria for semi-quantitative grading of the patients’ mental status. There were no standard mental state tests or questionnaires to evaluate hepatic encephalopathy that had been validated in Thai. A history of suspicious cognitive problems was screened in all the volunteers before initiating neurological examinations to rule out undiagnosed dementia. We did not use electroencephalography (EEG) to confirm encephalopathy due to limitations in our funding. Additionally, serum ammonia assays were not available in our hospital’s laboratory.
Neuroradiological assessment
Neuroradiological studies were not conducted on every patient due to limited funding. Patients whose physical examinations revealed apparent neurological deficits or patients with a prior history of cerebrovascular disease were sent for CT scans or magnetic resonance imaging (MRI) of the brain. Previous MRIs or CT scans of the brain that were conducted no more than one year before the initiation of this study were reviewed.
Statistical analysis
We used SPSS for Windows, standard version 11.5.0 (SPSS Inc., Chicago, IL, USA, 1989–2002) for statistical analysis. To determine the correlation between the outcomes and the independent variables for the movement disorder phenotypes, we used the chi-square or Fisher-exact test, with a p-value of less than 0.05 deemed to be statistically significant. Logistic regression was used to determine the significant risk factors associated with each movement and gait disorder phenotype. The analysis of the p-value, odds ratio (OR), and a 95% confidence interval (CI) were computed individually for all independent variables. Independent variables with a p-value of less than 0.10 were integrated for re-analysis with logistic regression to determine the final results for statistically significant risk factors that were related to movement disorder phenotype.
RESULTS
Intention tremor
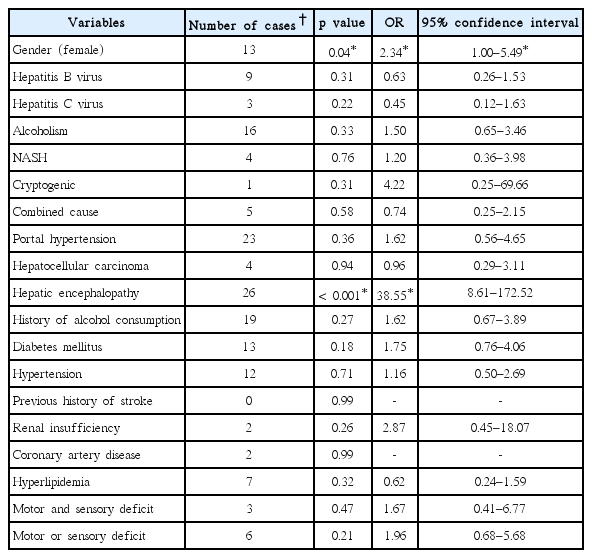
Of the 53 cirrhotic patients with intention tremors, four patients developed the tremor in their right hand, 30 patients developed the tremor in the left, and 19 patients developed the tremor in both hands. Male gender was a dominant trait among 48 subjects (48.5% within a gender), whereas the number of females was 5 (11.4% within a gender). The number of patients in each CTP score was 32 in CTP A, 17 in CTP B, and 4 in CTP C. The chi-square and Fisher-exact tests for the prevalence of intention tremors and all independent variables indicated the significance of the male gender (p < 0.001), alcoholic cirrhosis (p < 0.001), NASH (p = 0.01), and a history of alcohol consumption (p < 0.001). The univariate analysis of the different etiologies of hepatic cirrhosis and other independent variables are shown in Table 1.
The multivariate analysis with age-range adjustments confirmed the significance of the male gender and alcoholic cirrhosis to the prevalence of intention tremors at p = 0.002, OR = 5.70 of 95% CI 1.89–17.16, and p = 0.005, OR = 10.07 of 95% CI 2.02–50.14, respectively.
Bradykinesia
Bradykinesia was rated as 1 to 2 as referenced by the UPDRS part three criteria. There were 28 cases (15 males, 13 females). Most of the cases were in CTP B (15 patients), followed by 8 in CTP A and 5 in CTP C. Females were dominant, with a higher percentage than men (males 15.2%, females 29.5%). This gender difference yielded a significant effect (p = 0.04). The Chi-square and Fisher-exact tests for the prevalence of bradykinesia, and the different etiologies of hepatic cirrhosis revealed no significance. Portal hypertension and hepatocellular carcinoma also showed no significance. On the contrary, hepatic encephalopathy was statistically significant (p < 0.001). Analysis of all the confounding effects was inconsequential. The univariate analysis of factors associated with the prevalence of bradykinesia is illustrated in Table 2. Finally, the factors associated with bradykinesia included the female gender (p = 0.04, OR = 3.48, 95% CI 1.05–11.49) and hepatic encephalopathy (p < 0.001, OR = 43.32, 95% CI 9.30–201.51).
Parkinsonism
Fifteen cirrhotic patients who presented with Parkinsonism scored between 4 and 22 on the UPDRS motor scale (11 males, 4 females). Bradykinesia and cogwheel rigidity were rated as grade 1. Two patients showed mild postural instability, and two patients had resting tremors. Fourteen patients developed rigidity and bradykinesia bilaterally except for one patient who presented with right hemi-Parkinsonism. Ten patients were in CTP A, whereas 3 and 2 patients were in CTP B and CTP C, respectively. Statistical outcomes of the univariate analysis are shown in Table 3. From the multivariate analysis with age-range adjustment, the presence of hyperlipidemia (p = 0.04, OR = 0.11, 95% CI 0.01–0.95) and motor or sensory deficits (p = 0.02, OR = 5.05, 95% CI 1.28–19.79) were statistically significant.
Hyposmia was well established to be a premotor symptom of idiopathic Parkinson’s disease. Although hyposmia was not a movement disorder phenotype, it was included in the statistical analysis, especially in investigating its relationship to Parkinsonism. From the results of the assessment of the neurological deficits found in cirrhotic patients, there were 35 patients with hyposmia (29 male, 6 female). Male gender yielded an effect on the prevalence of an impaired sense of smell (p = 0.05, OR = 2.62, 95% CI 1.00–6.87). The univariate analysis did not show any significant relationship between hyposmia and other etiologies of cirrhosis or confounders. On the other hand, hepatocellular carcinoma and hepatic encephalopathy had a significant effect (p = 0.03, OR = 2.95, 95% CI 1.10–7.89, and p = 0.01, OR = 2.66, 95% CI 1.21–5.83). The association between hyposmia and Parkinsonism was analyzed. The initial results showed significant effects of p = 0.04, OR = 3.12, and 95% CI 1.04–9.36. However, the multivariate analysis with age-range adjustments confirmed that the hepatic encephalopathy and male gender were factors associated with hyposmia (p = 0.01, OR = 2.89, 95% CI 1.24–6.73, and p = 0.02, OR = 3.36, 95% CI 1.13–9.97).
Abnormal ocular movements
Twenty-nine patients presented with abnormal ocular movements (26 male, 3 female). Nystagmus and jerky pursuits were detected. The univariate analysis of these two characteristics is shown in Table 4.
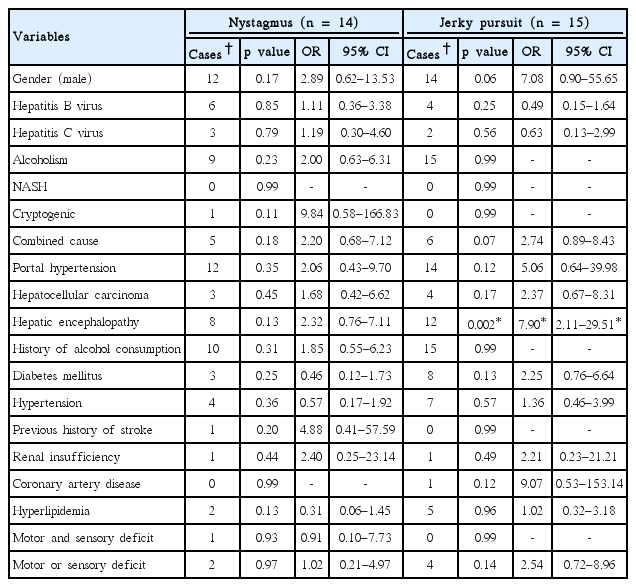
The univariate analysis of variables associated with the prevalence of nystagmus and jerky pursuits (n = 29)
Nystagmus
Fourteen patients (12 male, 2 female) had developed nystagmus. Horizontal nystagmus was the most common type. Three patients showed an upbeat-torsional nystagmus without evidence of impaired hearing function or abnormal cerebellar signs. Half of the patients with nystagmus were in CTP B. Although there was no association with nystagmus, this abnormal ocular movement was statistically significant with intention tremors at p = 0.03, OR = 3.47, 95% CI 1.09–11.00.
Jerky pursuits
The clinical feature of the disruption of smooth pursuit was observed in the conjugate eye movement of the horizontal axis. The saccade was preserved. There were 15 patients with alcoholic cirrhosis and a history of alcohol consumption. Seven patients were in CTP B, 6 patients were in CTP A, and 2 patients were in CTP C. Hepatic encephalopathy yielded an effect on the prevalence of jerky pursuits (p = 0.003, OR = 7.89, 95% CI 2.03–30.58) on multivariate analysis with age-range adjustments. This type of eye movement also was significantly related to bradykinesia (p = 0.04, OR = 3.21, 95% CI 1.03–9.94).
DISCUSSION
The subgroup analyses of each movement disorder phenotype have disclosed some interesting outcomes. The intention tremor was the most common movement disorder found in our study. The significant association between the prevalence of intention tremors and alcoholic cirrhosis shows that heavy alcohol consumption causes adverse effects on the cerebellum. Structural changes can be noticed not only in the cerebellum but also in the prefrontal cortex, pons, and thalamus of the brain of a chronic alcoholic. Neuronal cells of the cerebellar hemispheres, Purkinje cells and other cells of the molecular and granular layers of the cerebellar cortex and the cerebellar white matter are affected in patients with alcoholism [11]. It is proposed that the intention tremor appears when alcohol-induced neuronal degeneration occurs. As seen from the observations in our study, this kinetic tremor persists even in patients who no longer consume alcohol. This phenomenon probably reflects the irreversible neuronal damage in the cerebellar circuit. With the decompensation of hepatic function, the brains of cirrhotic patients may be more exposed to toxins than those of healthy people. From this point of view, we may be able to use the results from our study to campaign for alcohol cessation in Thailand as alcohol abuse continues to be a significant problem. Another finding related to chronic alcohol abuse is in the swaying and truncal tremors associated with atrophy of the anterior cerebellar vermis [12]. However, an ataxic gait in this study was seen in an insufficient number of cases for the subgroup analysis, so there is uncertainty about the factors associated with this gait impairment and its relation to other signs of cerebellar malfunction. Another finding that can possibly be caused by cerebellar dysfunction is nystagmus. Statistical analysis has shown a strong relationship between this eye sign to an intention tremor. We propose that both abnormal movements are common in alcoholic cirrhotic patients.
One interesting result is the strong relationship between bradykinesia and hepatic encephalopathy. The slowness of hand movements is visible and easily detected by assessing repetitive finger tapping. According to a previous report, this phenotype is defined as a fine motor impairment or as the slowing of finger movements. The pilot study by Butz et al. [13] showed that a decreased frequency of finger movements without a significant reduction of movement amplitude was found in cirrhotic patients who were at an early stage of hepatic encephalopathy. The Critical Flicker Frequency tool (CFF) was used for quantitative evaluation. CFF measurements focus on alternating between flexion and extension of the index finger while resting the other fingers. From a neurophysiological perspective, simple repetitive finger tapping is a process that requires the cooperation of the sensorimotor cortex and tactile-kinesthetic feedback. A decline in cortical function in conjunction with the stages of hepatic encephalopathy results in weak performance in finger movements [14]. Two experimental studies conducted in cirrhotic patients showed good reliability and accuracy of the CFF as a diagnostic tool for patients with mild hepatic encephalopathy [15,16]. Taking all previous results and the outcomes of our study into consideration, the slowness of finger tapping can be visually and sensitively detected in the mild or early stages of hepatic encephalopathy. Thus, we suggest finger tapping as a physical sign that coincides with the West-Haven criteria rather than as a negative myoclonus. It would be beneficial to assess this abnormal movement in an outpatient setting because physicians have limited time for physical examinations due to the high volume of patients. However, future research should be conducted with a larger sample size and a control group to confirm the sensitivity and specificity of the finger tapping impairment seen in patients with hepatic encephalopathy.
A jerky pursuit is another physical sign that has a strong association with hepatic encephalopathy. It can easily be detected on visual inspection. A typical eye pursuit movement in a healthy person occurs when the patient can see and carefully follow moving objects smoothly. With impairment, the eye movement distributes with a corrective saccade. Structural defects of the pursuit pathway can happen anywhere in the cerebral cortex, pons, and/or cerebellum. In hepatic encephalopathy, cerebral neurotransmission function is interrupted, and this results in a jerky pursuit [17]. An outcome of our study is in agreement with the results of a previous study that assessed the disruption of smooth pursuit eye movements in hepatic encephalopathy patients as a quantifiable value [18]. We also discovered a strong association between bradykinesia and jerky pursuits. These two signs are important indicators of cerebral malfunction. Therefore, fine motor impairment and the disrupted smooth pursuits should be considered useful and coordinated clinical signs for the detection of low-grade hepatic encephalopathy. However, due to the small numbers of volunteers in this subgroup, alcoholic cirrhosis and the history of alcohol consumption that may present in all cases with jerky pursuits cannot be interpreted using statistics.
The subgroup analysis of Parkinsonism shows the effects of the confounders that contribute to the feature. Some of these factors include hyperlipidemia, a previous history of cerebrovascular disease, and motor or sensory deficits. We summarize that Parkinsonism in this study is a consequence of peripheral neuropathy and lacunar infarctions. These two conditions are frequent incidences of metabolic diseases, especially DM. This outcome contrasts with previous studies that indicated Parkinsonism in nonWilsonian cirrhotic patients to be caused by CAHD and excessive manganese accumulation [1,8]. This inconsistent finding is possibly due to criteria that excluded severe cirrhotic cases with a high CTP score. The difference between the Parkinsonism seen in our study and some cases in previous reports is a low UPDRS motor score. Reports on CAHD have stated that Parkinsonism in non-Wilsonian cirrhotic patients was severe and carried a high UPDRS motor score (more than 30) with associative akinesia, rigidity, and a prominent postural instability. Their CTP scores were B or C, and neuroimages always revealed changes in the globus pallidus in T1-weighted images, supporting the hypothesis of manganese intoxication [8]. Various articles were published on Parkinsonism in patients who were awaiting liver transplantation [7,19]. All these reports represent findings from end-stage hepatic cirrhosis patients. However, no documentation during the initial state of hepatocerebral degeneration was provided.
Hyposmia is one of the premotor symptoms seen in idiopathic Parkinson’s disease. Thus, we analyzed its relationship to Parkinsonism. Although the outcome was negative in the multivariate analysis, we have discovered other possible clinical presentations of impaired cerebral function as seen in hepatic encephalopathy. In addition to the finger tapping and jerky pursuits, it is interesting to create a research methodology to approve the diagnostic value of hyposmia in hepatic encephalopathy. We also anticipate the progression of clinical symptoms in cirrhotic patients with hyposmia and Parkinsonism. Moreover, a multicenter study should be initiated to obtain more information on the clinical symptoms, investigations, and the pathology of Parkinsonism found in cirrhotic patients.
The limitations of our study are that there was a personal bias from one neurologist and no blinded control group. The lack of a laboratory testing, particularly in serum ammonia assays and EEGs, can be argued as leading to an overestimation of hepatic encephalopathy. The research team plans to conduct further studies to validate the psychomotor tests for hepatic encephalopathy for use in Thai patients. Additionally, the gender difference yields a significant effect on the prevalence of some movement disorder phenotypes. However, the researchers have not been able to explain this outcome. This finding may reflect the fact that the majority of non-Wilsonian hepatic cirrhosis in Thailand is seen in males because men are heavier drinkers than women. If alcoholic cirrhosis is included in the research, the number of men may always be higher than women for this population. This situation should explain the prevalence of intention tremors in males. In contrast, bradykinesia is dominant in females, and hyposmia has not shown to be associated with alcoholic cirrhosis; therefore, gender dominance continues to be an unknown factor.
Conclusion
Patients with hepatic encephalopathy show a pure, fine motor sluggishness and a disruption of ocular pursuit that presents with a horizontal nystagmus. Alcoholic cirrhosis has been closely associated with the development of an intention tremor.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Acknowledgements
This work was supported by the Faculty of Medicine, Srinakharinwirot University (grant number 433/2556).