Hereditary Cerebellar Ataxias: A Korean Perspective
Article information
Abstract
Hereditary ataxia is a heterogeneous disorder characterized by progressive ataxia combined with/without peripheral neuropathy, extrapyramidal symptoms, pyramidal symptoms, seizure, and multiple systematic involvements. More than 35 autosomal dominant cerebellar ataxias have been designated as spinocerebellar ataxia, and there are 55 recessive ataxias that have not been named systematically. Conducting genetic sequencing to confirm a diagnosis is difficult due to the large amount of subtypes with phenotypic overlap. The prevalence of hereditary ataxia can vary among countries, and estimations of prevalence and subtype frequencies are necessary for planning a diagnostic strategy in a specific population. This review covers the various hereditary ataxias reported in the Korean population with a focus on the prevalence and subtype frequencies as the clinical characteristics of the various subtypes.
INTRODUCTION
The word ataxia means the “absence of order,” and the term describes a clinical syndrome of incoordination caused by the dysfunction of cerebellum and its connected pathway. Currently, “ataxia” indicates specific disorders of the central nervous system in which the prominent phenotype is progressive ataxia. Hereditary cerebellar ataxias are a clinically, pathologically, and etiologically heterogeneous group of disorders [1,2]. In the past, several attempts have been made to classify hereditary cerebellar ataxias based on neuropathological features or clinical manifestations [3-5]. As molecular genetic methods have developed, genetic classification is more commonly used to study these ataxias. Hereditary ataxias are categorized by inheritance patterns in the following categories: autosomal-dominant, autosomal-recessive, X-linked, or mitochondrial mode of inheritance [6,7].
The prevalence of hereditary ataxia can vary among countries because of the founder effect [8-12]. Although the estimations of prevalence and subtype frequencies are necessary for planning the diagnostic strategy in a specific population, large epidemiological research about hereditary cerebellar ataxias is relatively rare in Korea compared to western countries. Various factors, including the fact that Korea is mostly a racially homogeneous nation, the shortage of family history, the low rate of typical phenotypes, and limited commercially available genetic tests, make it difficult to obtain an accurate diagnosis of these ataxias.
This review introduces the frequencies of hereditary ataxic disorders in Korea and the phenotypic features that have been reported by clinical, epidemiological and molecular genetic studies.
AUTOSOMAL DOMINANT CEREBELLAR ATAXIAS (ADCA)
Autosomal dominant cerebellar ataxias (ADCA) frequently represent spinocerebellar ataxias (SCAs). They are numbered according to the order of detection in the genetic locus. Recently, numerous novel SCA loci have been detected at a rapid rate and have extended to SCA 40 (Table 1) [13-15]. SCAs can be subdivided into four groups according to genetic mechanism, as follows [16]: polyglutamine disease due to the expansion of coronary angiography (CAG) triplet repeats [SCAs 1, 2, 3, 6, 7, 17 and dentatorubral pallidoluysian atrophy (DRPLA)], intronic disease (SCA8, 10, 12, 31, and 36), conventional mutation SCAs (SCA5, 11, 13, 14, 19/22, 23, 26, 27, 28, 29, and 35) and SCA with large duplications or deletions (SCA15 and 20).
The first group (polyglutamine disease) is more prevalent than the other forms of SCAs, and the mechanisms of the disease are well known [13]. Polyglutamine disease has several characteristic genetic features. The translated protein of the expanded triplet gene contains abnormally elongated glutamine repeats. The size of the expanded repeat is inversely correlated with the onset age, progression rate and clinical severity, which is referred to as anticipation [17,18]. This phenomenon is most striking in SCA7 and DRPLA due to lack of stabilizing intrusions (CAA/CAT) [17], and the phenomenon is less prominent in SCA6 because of the relatively small expansion and intergenerational stability [13]. In the CAG repeat disorders, genetic imprinting is also frequently observed, so paternal transmission is more likely to be associated with the occurrence of repeat expansions than maternal transmission of the expanded allele [18].
The most common subtype of intronic disease is SCA8, and its unique mechanisms have been recently reported [19,20]. SCA8 is thought to be caused by the bidirectional expansion of CTG/CAG leading to ribonucleic acid (RNA)-mediated toxicity and pathogenic polyglutamine tract formation. In contrast to polyglutamine disease, SCA8 shows expansions of the CTG repeat more frequently while maternal inheritance.
The clinical features of SCA overlap each other, and it is difficult to distinguish the subtypes of SCA with clinical manifestations. However, there are several distinguishable symptoms in each type [21]. SCA2 differs clinically from other types of SCAs because of the slow saccade and hyporeflexia [22]. SCA2 may present parkinsonism or amyotrophic lateral sclerosis [23,24], and cognitive impairment has also been reported for this subtype. The common features of SCA3, which is the most common subtype and called Machado-Joseph disease, are parkinsonism, dystonia, faciolingual myokymia and bulging eyes [16,25]. SCA6 is a pure cerebellar syndrome. The distinguishing feature of SCA6 is pronounced cerebellar oculomotor disturbance, including positioning and perverted headshaking downbeat nystagmus [26,27]. Patients with SCA7 experience a decrease in visual acuity and slow saccade, which are the predominant signs of the disease [28]. The most distinctive feature of SCA7 is the visual loss caused by pigmentary retinopathy. The phenotype of SCA17 is highly variable and complex. Various combinations of ataxia, chorea, cognitive impairment and parkinsonism make up the clinical manifestation of SCA17 [29].
DRPLA might be a dominant SCA. DRPLA is caused by expansion of abnormal CAG repeats in the DRPLA gene on 12p13.31 [30]. The pathological features of DRPLA are characterized by diffuse, marked brain atrophy, including the brainstem, dentate nucleus and pallidoluysian pathways. Although most patients with DRPLA have ataxia, they often have marked phenotypical variation according to the age of onset [31]. Childhood and adolescent-onset patients frequently have seizures and myoclonus, whereas adult onset patients typically have psychiatric problems, dementia and chorea [32,33]. We reported that cerebral white matter involvement could be the characteristic finding when imaging DRPLA, and these features might be helpful for the differential diagnosis between DRPLA and SCAs in the early stages of cerebellar ataxias [34].
Prevalence of ADCA
Although numerous epidemiological reports have attempted to determine the prevalence of ataxias in defined regions, an accurate estimation has yet to be well defined. The prevalence of the ADCAs is estimated to occur in approximately 1–5 people out of 100,000 people worldwide [35]. Dutch and Norwegian surveys [36] found the prevalence of ADCA to be 3.0 and 4.2 per 100,000, respectively. Of the ADCA, SCA3 is the most common subtype worldwide, followed by SCA1, SCA2, SCA6, and SCA7 [35]. However, the prevalence of subtypes of ADCA may vary by regional group due to the founder effects. SCA3, also called Machado-Joseph disease, was first introduced in Portuguese group from the Azores [37] and is commonly detected in Brazil [38], Portugal [39], Germany [40], China [41], and Japan [42]. SCA2 is the commonest subtype in Spain [43], southern Italy [44], India [45], and Cuba [46]. DRPLA is most prevalent in Japan and rare in North America [47].
SCAs in Korea
The only nationwide survey in Korea was performed from 2011 to 2012 using data from the Health Insurance Review and Assessment Service as well as data from the National Health Insurance Corporation [48]. According to this report, the prevalence rate of hereditary cerebellar ataxia patients in Korea is 4.99 patients/100,000 people. However, this study was performed based on diagnosis codes registered in the national statistical data rather than accurate clinical diagnoses made by experts. There is a possibility that ataxic diseases may have been underestimated or overestimated. Therefore, the exact prevalence of ataxic disorders and the frequency of the SCA subtype are still unclear.
Several single center-based studies exploring the frequency of SCAs have been published [49-52], and most of them revealed that SCA2 is the most common subtype in Korea, except for one study. Jin et al.[51] reported the prevalence of the SCA subtypes in the Korean population first. They found that SCA2 is most prevalent subtype followed by SCA6, and 3. 8 of 47 (17%) ataxic patients who were diagnosed with SCA did not have a definite family history. In 2003, Lee et al. [50] analyzed the frequencies of SCA subtypes in 253 patients who showed progressive ataxia, and the CAG triplet expansion was detected in 52 patients (20.6%). The most frequent subtype was SCA2, followed by SCA3, 6, 1, and 7. Interestingly, 4 out of 52 patients were misdiagnosed with multiple system atrophy (MSA) due to the negative family history and the presence of parkinsonism and dysautonomia. In contrast with previous results, Kim et al. [49] found that SCA3 was the most frequent subtype in their study. In that report, the authors performed a molecular analysis of SCA1, 2, 3, 6, and 7 in 76 individuals who showed signs of progressive ataxia. Thirty of the 76 individuals showed CAG expansions, and the most frequent subtype was SCA3 (15.8%) followed by SCA2 (14.5%). Among the 30 patients who were confirmed as SCA based on genetic confirmation, 4 (13.3%) were sporadic cases and 2 (6.7%) did not show an autosomal dominant inheritance pattern. Recently, in a multi-center analysis investigating the regional distribution of SCAs, 351 patients with SCA were identified [53]. The most frequent subtype was SCA2 followed by SCA3 and 6, and these three subtypes represented more than 70% of all cases in Korea. The subtype frequencies of SCAs varied considerably among the regions. In Seoul, Incheon and Gyeonggi, the subtype frequencies were similar to the subtypes in the entire Korean population. SCA3 was more frequent in the central areas, SCA2 was more frequently detected in the southern areas, and 93.6% (15 of 16) cases in Jeju were SCA6. These regional differences might reflect the founder effect or a mixing pattern with surrounding populations.
DRPLAs in Korea
Although several sporadic cases of DRPLAs have been reported in western countries, DRPLA appears to be very rare except in Japan. However, DRPLA is not uncommon in Koreans. According to previous reports, DRPLA is found at a rate of 3.4–4% of the hereditary ataxias in the Korean population [51,53].
AUTOSOMAL RECESSIVE CEREBELLAR ATAXIAS (ARCA)
There are over 55 disorders with an autosomal recessive inheritance pattern, and the “big six” recessive ataxias include the following diseases: ataxiatelangiectasia (AT), Friedreich ataxia (FRDA), ataxia-ocular motor apraxia types 1 and 2 (AOA), autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS), and POLG-related disorders.
Prevalence and clinical features of ARCA
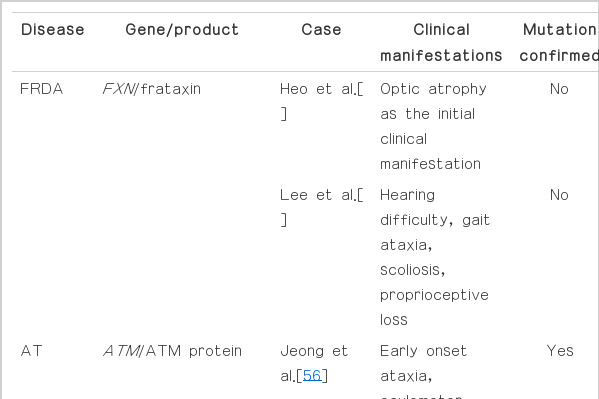
Hereditary ataxias with autosomal recessive inheritance account for 3:100,000 cases worldwide. In Korea, patients with recessive ataxia are rare (Table 2). Although FRDA is the most common genetic ataxia in Caucasians (one-third of recessive ataxias are FRDA), it is extremely rare in east Asia. In Korea, a few cases of FRDA were reported, but there was no genetic confirmation [54,55]. AOA is the most common recessive ataxia in Japan, the second most common recessive ataxia in Portugal (representing approximately 4% of recessive ataxias), but AOA is also not reported in Korea. Therefore, this article will concentrate on recessive ataxias reported in Korea.
Ataxia telangiectasia
AT is caused by mutations in the ATM gene, and it is the second most common recessive ataxia worldwide. AT is characterized by progressive ataxia beginning in early childhood and telangiectasia. Clinical manifestations include cerebellar ataxia, oculomotor apraxia, choreoathetosis and dystonia. Physical examination reveals characteristic telangiectasias of the conjunctivae, nose, palate and cubital fossae. Patients with AT experience frequent respiratory infections due to immunodeficiency and they run a risk of malignancy because of radiosensitivity.
The prevalence of AT is estimated to be between 1 in 40,000 people and 1 in 300,000 people depending on ethnic specificity. Although AT is the most common cause of ataxia in childhood in most populations, it is rarely found in Korea. Four cases of AT have been reported in Korea, and only 2 cases were genetically confirmed with gene sequencing [56-59]. We reported a delayed diagnosis of AT with 2 novel splicing mutations in the ATM gene [56] because of subtle telangiectasias of conjunctivae. This case emphasized the importance of clinical suspicion of AT even though it is a rare and atypical clinical manifestation in Korea.
Cerebrotendinous xanthomatosis
Cerebrotendinous xanthomatosis (CTX) is a rare autosomal recessive form of xanthomatosis caused by deficiency of the enzyme sterol 27-hydroxylase (CYP27), which results in the accumulation of cholesterol and cholestanol. CTX is caused by mutations in the CYP27A1 gene, which is located on chromosome 2q33-qter and manifests childhoodonset cataracts followed by adult-onset tendon xanthomas and progressive neurologic symptoms, including cerebellar ataxia, dystonia, cognitive impairments, and seizures.
There have been two cases of CTX diagnosed by gas chromatography [60] and gene confirmation [61]. In both cases, the typical phenotype developed in early childhood or adulthood, but the diagnosis was delayed for approximately 10 years. Metabolic issues can be improved by treatment with chenodeoxycholic acid. Furthermore, treatment can prevent neurological symptoms, so early diagnosis and treatment is important and essential for CTX.
Autosomal recessive spastic ataxia of Charlevoix-Saguenay
ARSACS was introduced in Quebec, Canada, and is restricted to this area. The phenotype of ARSACS was very homogeneous and showed a typical triad of leg spasticity, peripheral neuropathy and infantonset cerebellar ataxia. After detecting the accountable gene, SACS, ARSACS has also been reported outside of Quebec in countries including Japan, Europe, and North Africa. Now we know that ARSACS is not an endemic disease in Quebec. Basically, the clinical manifestations of ARSCAS were reported to be very homogeneous in Quebec, and non-Quebec patients usually present with atypical phenotypes.
In Korea, only one case of ARSACS has occurred. The patient showed the classic triad of early childhood-onset cerebellar ataxia, peripheral neuropathy and pyramidal tract signs such as spasticity, abnormal reflexes and the loss of the ability to perform fine motor movements. The patient also revealed typical MRI findings, including bilaterally symmetrical, parallel, linear hypointensities in the pons on T2 and T2-fluid attenuated inversion recovery magnetic resonance image (MRI) sequences as well as yellow streaks of hypermyelinated fibers radiating from the edges of the optic fundi in the retina (unpublished data).
X-LINKED ATAXIA
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a recently recognized disorder. Fragile X syndrome is caused by FMR1 gene mutation, and Fragile X is the most common cause of genetic mental retardation. In Fragile X patients, the CGG trinucleotide sequence expands more than 200 times, and this area is usually hypermethylated, which usually leads to gene silencing and the absence of the Fragile X mental retardation protein (FMRP). The mental retardation is caused by the lack of FMRP in neurons in these patients. Expanded CGG sequences between 55 to 200 triplets are called premutations. These alleles produce increased levels of messenger RNA (FMR1 mRNA) that are up to eightfold higher than normal as well as a decreased range of FMRP. Individuals with a premutation do not manifest mental retardation, which is classical phenotype of Fragile X syndrome. However, these individuals exhibit a unique phenomenology typified by progressive ataxia and tremors, which is called FXTAS.
The only case of FXTAS was reported very recently in Korea [62]. A case of a 75-year-old male with resting tremors as the initial symptom was confirmed as FXTAS by detecting FMR1 gene permutations (136 repeats) using polymerase chain reaction and a southern blot. His MRI showed typical imaging features of T2 high signal intensity in the middle cerebellar peduncles as well as marked cerebellar and cerebral atrophy.
CLINICAL IMPLICATIONS
The diagnosis of hereditary ataxias should be considered based on ethnicity and region, and epidemiological data can help to determine if genetic sequencing should be performed. Although we cannot estimate the absolute prevalence and frequency of cerebellar ataxia, we suggest a rough estimate of the hereditary ataxia in Korean population. SCA2, 3, and 6 are the major disorders among the dominant SCAs in Korea. Overall, SCA2 is the most common cause of inherited ataxia, regardless of the inheritance pattern. DRPLA is rare outside of Japan, but it is not uncommon in Korea. The reports of cerebellar ataxias with autosomal recessive and X-linked inheritance patterns have been extremely rare [63,64].
We need to address the algorithm for the diagnosis of hereditary ataxia in the Korean population. In cases without a family history, secondary causes include alcoholic cerebellar degeneration, vitamin deficiency, drugs, toxic materials, strokes, tumors, infections, autoimmune diseases, paraneoplastic syndrome or demyelinating disease. Ataxias with secondary causes usually have an acute or subacute-onset and rapid progression. After ruling out the secondary causes of ataxia, a follow-up period is needed to confirm the diagnosis of MSA or sporadic adult onset ataxia with unknown origin (SAOA). Another point that we should consider is the ambiguity of the family history in genetic ataxias. In previous studies, a considerable number of patients who had the expanded pathological allele had a negative family history. Therefore, even if the patients have an inaccurate, ambiguous or negative family history, hereditary ataxia should be considered. One more important point to remember is that patients with SCA6 may not have a family history. The age of onset in SCA6 is higher than other types of SCAs. Early deaths or inappropriate diagnoses of parents are important factors to consider, and a de novo mutation may be another possibility.
Additionally, screening of SCA genes in patients with adult-onset chronic progressive cerebellar ataxia (who do not meet the criteria for the MSA-cerebellar type) would be needed regardless of family history, even if there were a less conclusive family history for confirming the inheritance pattern and overall detection rate of ADCA.
For early onset progressive ataxia (< 30 years), even though the patients do not require a family history, we should consider a possibility of hereditary ataxia, especially for ARCA. When ARCA is suspected, fundus examination and careful physical examination should be performed to find telangiectasia, xanthoma, limb deformity or retinitis pigmentosa. Additionally, we should find the clues by performing blood smears as well as tests for vitamin E, alpha-fetoprotein, cholesterol, thyroid function, immunological disorders, lactate/pyruvate, nerve conduction and brain MRI. If clinical or laboratory clues indicate specific disease, genetic sequencing is needed to confirm the diagnosis.
Unless certain suggestive clinical signs are shown, we emphasize that the screening for SCAs is recommended because of the high rate of SCA in the Korean population.
In cases with autosomal dominant inheritance patterns, clinical features become evident at approximately 35 years of age. SCA1, 2, 3, 6, 7, 8, and 17, which are most frequent subtypes in Korea, should be screened for first. If the results of those tests are negative, careful follow-up is needed to find evidence of MSA or SAOA.
It is true that ARCA is rare in Korea. Most centers in Korea perform screenings for the SCA and DRPLA gene for patients with adult-onset chronic progressive ataxia, and the possibility of underestimation for ARCA cannot be excluded. The paucity of full sequencing for ARCA and homogeneous genetic background might explain the rarity of ARCA in Korea. Awareness of the variable clinical features of ARCA (Table 3) might be helpful for clinicians when selecting candidates for further genetic evaluations. Case control studies and epidemiological studies, including genetic sequencing of ARCA, are needed in Korea.
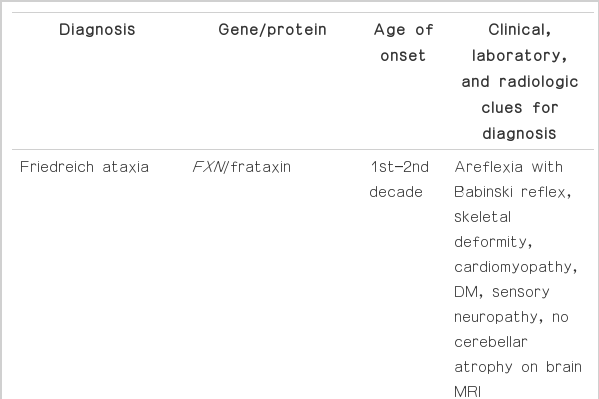
Clinical, laboratory and radiological clues for the diagnosis of autosomal recessive cerebellar ataxias
The availability of novel genetic technologies for both research and diagnostic laboratories is rapidly developing, and this advance will facilitate rapid progress in this field. In the near future, more efficient diagnosis and the identification of many novel forms of ataxic diseases are expected.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Acknowledgements
This work was supported by a Samsung Medical Center grant (SMO1131541 and CB13152).
We are grateful to Prof. Seong-Beom Koh, Han-Joon Kim, Kyum-Yil Kwon, and Dr. Gwanhee Ehm for providing information about rare cases.