Orthostatic Hypotension and Cognitive Impairment in De Novo Patients with Parkinson’s Disease
Article information
A relationship between orthostatic hypotension (OH) and cognitive dysfunction in Parkinson’s disease (PD) has been recently investigated [1-3]. Most of the previous studies were performed with patients taking anti-parkinsonian medications, and one study was performed in patients before medication included patients with dementia. The authors studied OH in drug-naïve and non-demented patients with PD [4]. To find the relationship between cognitive dysfunction and OH, we performed an additional analysis of the association between cognitive function and OH in our cohort of PD patients [4].
The clinical characteristics of the subjects (45 drug-naïve PD patients, 17 men and 28 women, age of 63.8 ± 10.1 years, disease duration of 1.3 ± 1.1 years) and assessment of OH were described in a previous report [4]. Briefly, OH was measured by monitoring systolic and diastolic blood pressure (BP) using a tilt table. After a 10 minute period of lying down, the table was tilted to 60° upright, and the BP was recorded at the first, third, and fifth minutes after tilting. OH was defined as a drop in systolic BP ≥ 20 mm Hg or in diastolic BP ≥ 10 mm Hg at any time following being uprighted [2]. A detailed cognitive assessment was performed using the Seoul Neuropsychological Screening Battery (SNSB) [5]. Abnormal results of the SNSB were determined by a comparison with the results of a standard control group (less than 1 standard deviation) in each of six domains (attention, language, calculation, visuospatial function, memory, and frontal/executive function). SPSS version 19 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical calculations. The Mann-Whitney and chi tests were employed to analyze intergroup difference. Statistical significance was declared at the p < 0.05 level.
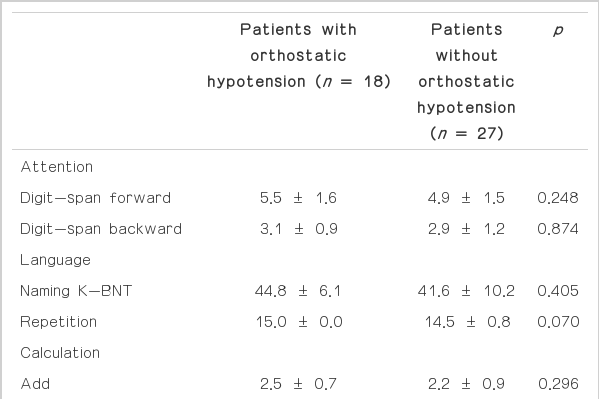
The results showed that nearly all patients (43/45, 95.6%) had an abnormality in at least one cognitive domain. Thirty five patients (77.8%) showed multiple domain abnormalities. Because all the participants did not show any impairment in the activities of daily living due to cognitive dysfunction, those patients with abnormal results could be classified as having mild cognitive impairment (MCI). Frontal/executive function was the most frequent abnormal domain (71.1%), followed by memory (66.7%), calculation (51.1%), and attention (44.4%). Abnormal results in patients with OH were more common in attention, memory, and frontal/executive function tests compared with those in patients without OH, but the differences were not significant. The overall performance of the patients showed no differences in terms of OH, but in the memory domain, the OH group showed significantly poorer performance in the recognition test of verbal memory than the normotensive group (Table 1). A similar non-significant trend was observed in the recognition test of visual memory. Discrimination tasks of recognition were also decreased in the OH group, and those differences became clear when the values were switched to percentiles of the general population (data not shown). There was no difference in the results of the cognitive function tests for those patients without any other comorbid disorders (data not shown).
Several cognitive domains were reported to be associated with OH in PD, such as attention, visuospatial function and verbal memory. Memory was the most consistent domain throughout the reports, and in particular, verbal memory was the only domain associated with OH in the patients before medication [2]. Our results also revealed an association between OH and verbal memory dysfunction, but with respect to recognition rather than recall. Because recognition has been known to be preserved in PD patients, the poor performance in the recognition task by PD patients with OH would suggest that OH could contribute to temporal cortical dysfunction [6]. OH results in transient or sustained cerebral hypoperfusion and is associated with the presence of white matter lesions [2]. However, another study failed to find any difference in white matter lesions between patients with and without OH [3]. Although the role of ischemic burdens in OH has still not been established, OH could be a potential therapeutic target for the prevention of dementia in PD patients and warrants early detection and appropriate management. However, cognitive dysfunction and autonomic dysfunction, such as OH, can be a clinical marker for more extensive Lewy body pathology. Further studies should allocate the OH in the pathological context of PD and the clinical implications.
There are several limitations in this report. We excluded PD patients with dementia, but detailed cognitive assessment revealed cognitive dysfunction in nearly all PD patients (43/45) compared with the age- and education-matched standard population. Although the prevalence of MCI in PD patients varies considerably depending on the report, there could be a selection bias in the setting of a tertiary referral hospital [7]. Another limitation is the clinical diagnosis of the patients. For diagnostic accuracy of PD, we enrolled PD patients who were followed up more than six months after the diagnosis of the patients. However, patients with atypical parkinsonism, especially multiple system atrophy and diffuse Lewy body disease, may have been included in our study. We did not control the medication for comorbid disorders, such as hypertension and benign prostate hypertrophy, which might have influenced the BP results.
In conclusion, we found that OH was associated with cognitive dysfunction, especially the verbal memory task. The early detection and appropriate management of OH could have potential for the prevention of cognitive deterioration in patients with PD.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.