Normal Cerebellar Metabolism in a Patient with Superficial Siderosis
Article information
Dear Editor,
In many cases with hereditary and non-hereditary cerebellar ataxia, cerebellar dysfunctions, even in the early phase, are observed in the resting state. It has been also hypothesized in patients with superficial siderosis that accumulated hemosiderin-induced parenchymal damage in the cerebellum may lead to reduced neuronal metabolism, resulting in functional deterioration of the cerebellum. However, there are no data about the regional cerebral metabolism and perfusion reflecting neuronal activity in superficial siderosis. Here, we report a case of superficial siderosis that had the typical findings of the disease and the results of positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG).
A 66-year-old woman complained of a 4-year history of a slow progressive unsteadiness of gait and hearing difficulty. The patient also had subjective forgetfulness and depressive mood. There was no family history of similar symptom and no prior history of head trauma, intradural surgery, neck or backache, bladder disturbance, stroke or transient ischemic attack. On examination, her gait was markedly ataxic, with a tendency to fall. There was no eye movement limitation or other cranial nerve involvement, except for bilateral hearing impairments. There were no extrapyramidal signs, such as bradykinesia, rigidity or dystonia. Tendon reflexes were hyper-reactive, and the Babinski sign was elicited on the right side. The patient scored 32/100 on the international co-operative ataxia rating scale (postural and gait disturbance 20/34, limb ataxia 12/52). The results of our routine investigations, including hematological and biochemical screening, thyroid function test, levels of vitamin B12 and folic acid, tests for antinuclear factor, HIV and VDRL and measurements of the serum ceruloplasmin and copper levels, were normal. Serum iron was decreased to 39 mcg/dL (normal 50–150), and serum ferritin levels were elevated to 166.8 ng/mL (normal 13–150). Magnetic resonance imaging (MRI) of the brain showed marginal hypointensity involving the brain stem, cerebellum and upper cervical cord in T2-weighted images (Figure 1A). Spine MRI also demonstrated hypointensity along the superficial surface of the whole spinal cord on T2-weighted images (Figure 1B). An intracranial angiography showed no aneurysms or vascular malformations, and a brain PET using FDG showed no definite regional metabolic abnormalities of the cerebellum (Figure 1C). Cognitive evaluations, including the Seoul Neuropsychiatric Battery, showed no definite abnormality. Autonomic functions with the head-up tilt test, sympathetic skin response, heart rate response to deep breathing and Valsalva maneuver were normal. A smell identification test was also normal. Audiometry demonstrated bilateral sensory neural hearing impairment.
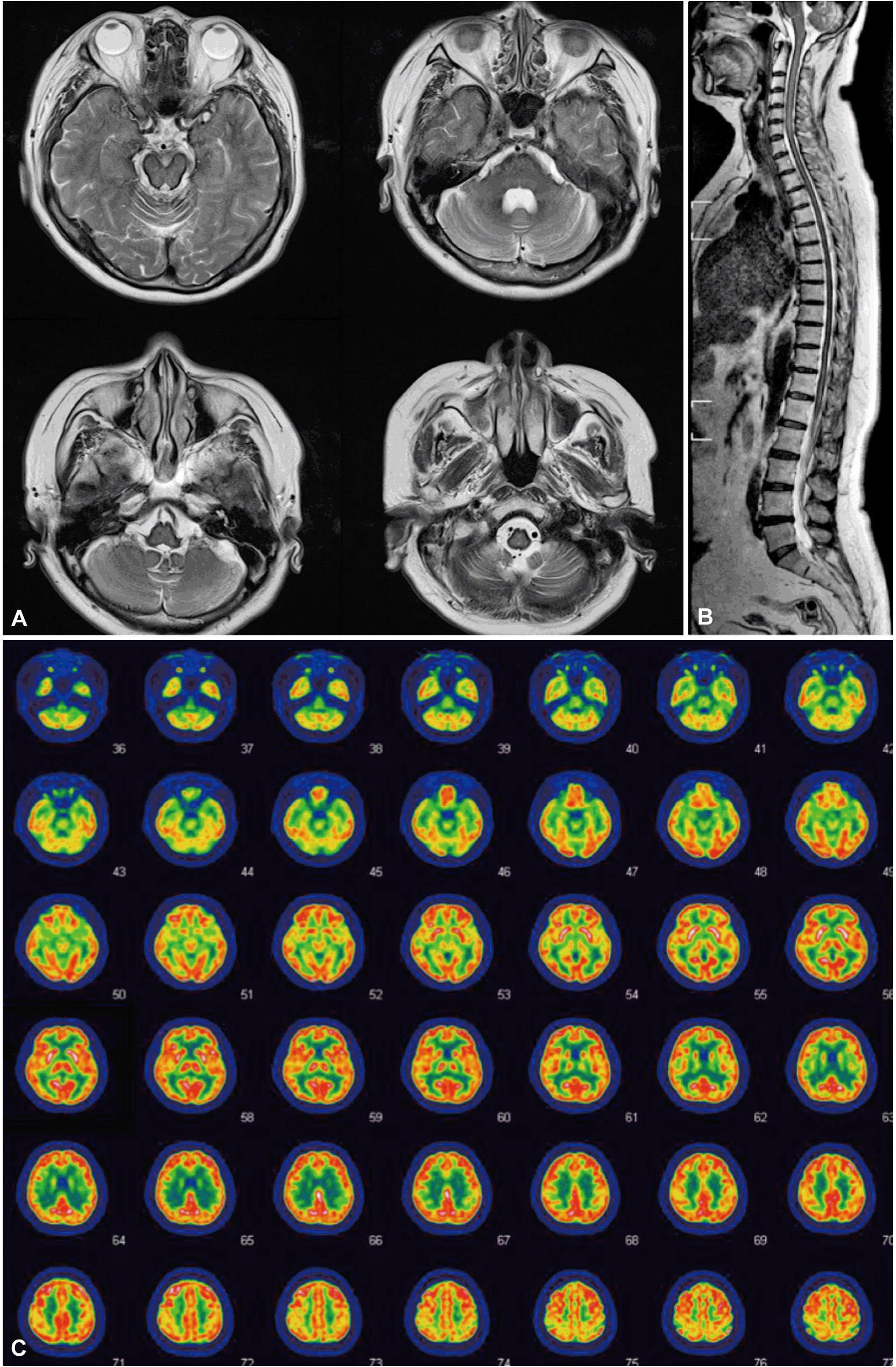
T2-weighted axial brain magnetic resonance imaging (A) and whole spine T2-weighted sagittal magnetic resonance imaging (B) showing a rim of dark signal intensity along the superficial surface of the brain stem and spinal cord, suggesting hemosiderosis. Positron emission tomography using 18F-fluorodeoxyglucose revealed normal metabolism in both cerebellar hemispheres (C).
The patient was treated with an iron-chelating agent, deferasirox 125 mg/day. Within 6 months of medical therapy, the gait disturbance was not improved, but there were no adverse effects.
Superficial siderosis is a distinct clinical syndrome caused by chronic slow or repeated subarachnoid hemorrhage into the subarachnoid space, leading to hemosiderin deposition on the subpial surfaces of the central nervous system (CNS) [1]. The deposition of hemosiderin is associated with gliosis, neuronal loss and demyelination, resulting in parenchymal damage, especially in the cerebellum, brainstem and spinal cord. Superficial siderosis is characterized by sensorineural deafness (95%), cerebellar ataxia (88%), and pyramidal signs (76%); other features include dementia (24%), bladder disturbance (24%), anosmia (at least 17%), anisocoria (at least 10%) and sensory signs (13%) [2]. The cardinal features of the disease are deafness and cerebellar ataxia, which occur in approximately 90% of cases. Thus, the predominant phenotype resembles spinocerebellar degeneration with progressive ataxia, hearing loss, and corticospinal tract signs. However, the exact mechanism of superficial siderosis is still unknown. In an animal model, the earliest microglial response in the cerebellar molecular layer was found to be hyperplasia and hypertrophy, as displayed by ferritin-reactive cells. This early apoferritin response most likely occurred due to the presence of heme, rather than iron, in the cerebrospinal fluid and subpial tissue. Ferritin biosynthesis is accelerated when the ferritin repressor protein is dissociated from the ferritin mRNA. A specific antiserum ferritin repressor protein localizes predominantly to astrocytes, including Bergmann glia, and it is proposed that the abundance and proximity of ferritin repressor protein-immunoreactive Bergmann glia and ferritin-containing microglia in the cerebellar molecular layer permit a prompt cellular interaction in the conversion of heme to ferritin and ultimately hemosiderin [3].
Almost all patients have a distinct MRI appearance. The cerebellum and brainstem were encompassed by a dark, hypointense rim on T2-weighted images. Cerebellar atrophy is present in all cases, and spinal cord MRI is remarkable for a diffuse T2- weighted hypointensity of the pial surface of the cord. A similar rim of T2-hypointensity along the nerve roots of the cauda is relatively commonly observed, at times with clumping of the nerve roots, suggesting arachnoiditis. An intraspinal fluid-filled collection of variable dimensions is frequently observed on spine MRI [4]. A prior history of trauma (at times trivial) or intradural surgery (commonly involving the posterior fossa) may be present. Treatment for superficial siderosis is difficult because even after operatively ablating the source of subarachnoid bleeding, the disease will likely progress because the hemosiderin deposition already present by the time of presentation overwhelms the patient’s ability to clear it [5]. There is a case report that progression may have been retarded over 2 years using an iron-chelating agent such as trientine dihydrochloride [6], but other attempts to use an iron chelator to treat superficial siderosis have failed. There is a recent encouraging report of an effective therapy using deferiprone, a lipid-soluble iron chelator, which has been shown to easily cross the blood-brain barrier and chelate iron in the CNS. A pilot safety trial of deferiprone was successfully completed, and a prospective placebo-controlled observational study is ongoing [7]. We performed brain PET using FDG. Considering her 4-year history of progressive ataxic gait, we expected decreased cerebellum glucose metabolism. However, contrary to our anticipation, the results of brain FDG PET showed no definite metabolic abnormality in the brain. This result suggests that the symptoms and signs of superficial siderosis may be due to other causes than decreased glucose metabolism of the cerebellum. Considering the very rare incidence of this disease, generalization of this functional brain imaging is very limited. Another case study or longitudinal follow-up will be needed to ascertain the neuronal activity and its association with disease progression.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.