Articles
- Page Path
- HOME > J Mov Disord > Volume 16(3); 2023 > Article
-
Original Article
Cervical proprioception in Parkinson's disease and its correlation with manual dexterity function -
Özlem Menevşe1

 , Büşra Kepenek-Varol1
, Büşra Kepenek-Varol1 , Murat Gültekin2,3
, Murat Gültekin2,3 , Sevil Bilgin4
, Sevil Bilgin4
-
Journal of Movement Disorders 2023;16(3):295-306.
DOI: https://doi.org/10.14802/jmd.23039
Published online: July 3, 2023
1Department of Physiotherapy and Rehabilitation, Faculty of Health Sciences, Nuh Naci Yazgan University, Kayseri, Turkey
2Department of Neurology, Faculty of Medicine, Erciyes University, Kayseri, Turkey
3Department of Neurology, Faculty of Medicine, İstanbul Atlas University, İstanbul, Turkey
4Department of Physiotherapy and Rehabilitation, Faculty of Physical Therapy and Rehabilitation, Hacettepe University, Ankara, Turkey
- Corresponding author: Özlem Menevşe, MSc, PT Department of Physiotherapy and Rehabilitation, Faculty of Health Sciences, Nuh Naci Yazgan University, Küme Evler, Kocasinan, Kayseri 38170, Turkey / Tel: +90-352 324 0000 ext. 5358 / Fax: +90-352 324 0004 / E-mail: menevseozlem@gmail.com
Copyright © 2023 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,955 Views
- 110 Download
ABSTRACT
-
Objective
- Cervical proprioception plays a crucial role in posture and movement control. This study aimed to determine the relationships of cervical proprioception, cervical muscle strength and endurance with manual dexterity and hand strength in individuals with idiopathic Parkinson’s disease (PD).
-
Methods
- Twenty individuals with PD (mean age: 63.9 years) and 20 healthy individuals as a control group (mean age: 61.9 years) were recruited. Cervical joint position error (JPE), static endurance of neck muscles, activation of deep cervical flexor muscles (Craniocervical Flexion Test, CCFT), manual dexterity (Purdue Pegboard Test, PPT), cognitive and motor tasks of the PPT, finger tapping test (FTT), pinch strength, and grip strength were assessed.
-
Results
- Cervical JPE was significantly higher in individuals with PD than in controls (p < 0.05). The strength and endurance of the cervical muscles were significantly decreased in individuals with PD (p < 0.05). Cervical JPE measurements were negatively correlated with PPT, cognitive and motor tasks of the PPT in individuals with PD (all p < 0.05). The endurance of cervical flexor muscles was negatively correlated with PPT and cognitive PPT scores in the PD group (p < 0.05). In addition, a significant positive correlation was found between cervical flexor endurance and hand strength in the PD group (p < 0.05).
-
Conclusion
- Cervical proprioception and the strength and endurance of cervical muscles decrease in individuals with PD compared to healthy individuals. Impairment of cervical proprioception appears to be associated with poorer upper extremity performance. Detailed evaluation of the cervical region in PD may help determine the factors affecting upper extremity function.
- Parkinson’s disease (PD) is a progressive neurodegenerative disease that involves dopaminergic neurons in the basal ganglia [1]. PD, characterized by disruptive movement disorders, causes progressive limitations in upper extremity activities such as reaching, grasping, and fine motor skills from the early stages [2]. Although the exact cause of manual dexterity loss is unknown, fine dexterity disorders can be observed in patients, especially due to bradykinesia, hypokinesia, tremor, and rigidity [2,3]. Due to bradykinesia, reaching out, grasping, and manipulating become difficult [4]. Reduction in handwriting size (micrography), difficulty with activities such as buttoning and brushing, and a decrease in arm swing occur [1,2]. Impairment in finger torque production and control may reduce manipulation ability, while limb kinetic apraxia may hinder daily living skills [3,5]. All these symptoms lead to the deterioration of upper extremity functions and, as the disease progresses, limitations in activities of daily living and a decrease in quality of life [4].
- Proprioception is a sense that includes the perception of joint position and movement and provides afferent input to the central nervous system for motor planning [6,7]. Accurate motor movement is ensured by well-integrated and intact information from the visual, vestibular, and somatosensory systems, especially the proprioception system [7]. Impairment of proprioception leads to changes in the accuracy and timing of motor commands and affects movement control [6]. Cervical proprioception is defined as the perception of the position of the head or neck in space and requires a complex interaction between afferent and efferent receptors. Muscle spindles are the main proprioceptors in the cervical region. In addition, Golgi tendon organs, cutaneous receptors, and joint receptors provide afferent information [8]. Cervical proprioceptors make central and reflex connections with vestibular, visual, and postural control systems [7]. Especially in deep cervical muscles, the density of muscle spindles is extremely high [9]. Therefore, the deep cervical muscles are thought to provide stability and postural support by fine-tuning cervical motion [6]. Normally, proprioceptive information from cervical muscle spindles is integrated into the central nervous system to control head position, orientation, and body posture. A change in the afferent information-providing structures of the cervical region also changes the information transmitted to the central nervous system [9].
- The relationship between cervical proprioception and upper extremity proprioception in healthy individuals and in various disease groups has been investigated [10-14], but to the best of our knowledge, there has been no such study in PD. Investigating the relationship between the cervical region and upper extremity function will be useful in determining the factors affecting the performance of the upper extremity in PD. Therefore, this study aimed to investigate how cervical proprioception, cervical muscle strength and endurance are related to manual dexterity and hand strength in individuals with PD. This study tested two hypotheses: 1) The sense of proprioception in the cervical region and the strength and endurance of the cervical muscles are weaker in PD than healthy individuals. 2) The sense of proprioception in the cervical region and the strength and endurance of the cervical muscles are correlated with manual dexterity and hand strength in Parkinson’s patients.
INTRODUCTION
- Study design
- In this cross-sectional study, 20 individuals with PD and 20 healthy age- and sex-matched individuals as controls were evaluated for cervical proprioception, cervical muscle strength and endurance, manual dexterity, and hand strength. This study was conducted between March 2022 and August 2022. The study was conducted in accordance with the Helsinki Declaration. For the study, ethics approval was received from Erciyes University Clinical Research Ethics Committee (date 09.03.2022, No. 2022/218). A written informed consent form was received from each participant.
- Participants
- Patients who were diagnosed with idiopathic PD according to the United Kingdom Brain Bank Parkinson’s Disease criteria, were aged > 40 years, had Hoehn-Yahr Staging < 3, had a Mini-Mental State Examination (MMSE) Score ≥ 24, received only oral medical treatment, and had no change in medical treatment for PD in the last month were included in the study. Patients who received device-assisted therapy; received apomorphine therapy; took medications that could affect cognitive function (such as antidepressants); had orthopedic, neurological, and vestibular problems other than PD; or had hearing and speech problems were excluded from the study. All patients with PD were evaluated in the “on” (drug-effective) period, when they were not currently receiving rehabilitation (they were only followed up with an intermittent home program). Healthy individuals with similar characteristics to the PD group in terms of age and sex, had a Mini-Mental Test Score ≥ 24 and had no orthopedic, neurological, or vestibular problems were included as a control group.
- Evaluations
- The activities of daily living and motor subscores of the Unified Parkinson’s Disease Rating Scale (UPDRS) were evaluated. Items 20 (resting tremor), 21 (action or postural tremor), 22 (rigidity) and 23 (finger tapping) were accepted as symptoms of PD with associated hand function. Item 28 (posture) was used to assess postural change. Each item was scored between 0 and 4, a lower score indicating better status.
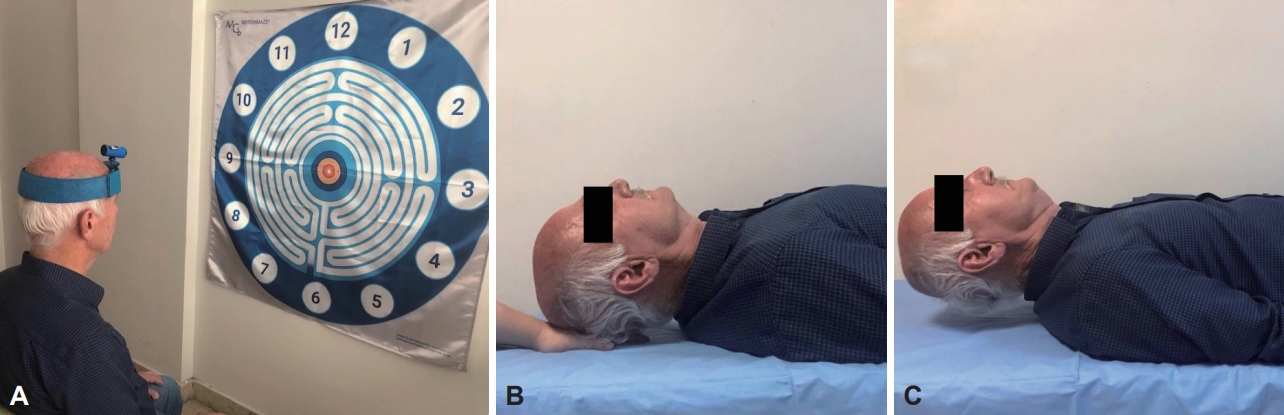
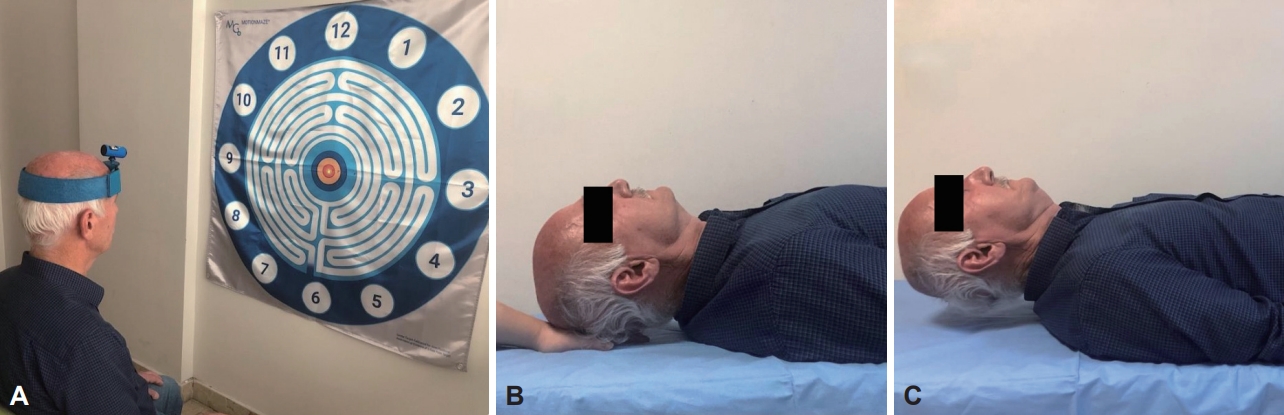
- Flexion, extension, right rotation, and left rotation joint position error (JPE) were evaluated with a laser kit (Motion Guidance Clinic Kit, Motion Guidance LLC, Denver, CO, USA) for the proprioception sense of the cervical region. The laser kit was placed on the head of the participants, and they were asked to sit in a comfortable position 90 cm from the target point (Figure 1A). After closing their eyes, they were asked to indicate the neutral position of the head and memorize it to return after movement, and this point was recorded as a reference. In the next step, they were asked to return to the initial reference position with maximum accuracy, without any speed instruction, after performing a maximum cervical movement, and to stop and say “OK” when they thought that they had reached the target. The point at which the light beam stopped was recorded as the overall error, measured in centimeters (cm), relative to the center of the previously recorded target. Five repetitions were performed for each direction, and their average was taken [15].
- Craniocervical Flexion Test (CCFT) was used to evaluate the control of deep cervical flexor muscles. A pressure sensor (Stabilizer, Chattanooga, TN, USA) was placed between the earlobe and the chin projection while the participant was in the supine hook lying position. The participants were asked to perform craniocervical flexion movement with the feedback of the pressure sensor increasing in 2 mmHg increments starting from 20 mmHg and ending at 30 mmHg. The activation score and performance index were recorded. The pressure level that could hold ten repetitions for 10 seconds was recorded for the activation score. The performance index was calculated according to the number of repetitions at the pressure level that they could hold for 10 seconds [16].
- The participants were asked to raise their head while lying in the supine hooked position, and the head weight was supported by the hand of the evaluator under the occiput in this position (Figure 1B). They were then asked to bring their head slightly to the craniocervical flexion position. After learning the position, the time they were able to maintain the craniocervical flexion position by slightly lifting their head from the evaluator’s fingers was recorded (Figure 1C). This was all done a second time after 5 minutes of rest, and the best value was accepted as the result [17].
- The participants were fixed to the bed at the level of the T6 vertebrae while lying in the prone position with the arms at the side and the head and neck out of the bed. A laser kit was placed on their head to observe the deviation in head position during the test. In this position, they were asked to lift the weight (4 kg for males, 2 kg for females) attached by Velcro just above the ears. The test began with the participants bringing their head to a slightly craniocervical flexion against the weight, and the time held was recorded. With reference to the displacement of the laser beam, the test was terminated when the head deviated more than 5° from the initial position for a minimum of five seconds once or when it slightly deviated more than five times. The expected target time to complete the test was 600 seconds [18].
- The Purdue Pegboard Test (PPT) is used to assess manual dexterity. In the first three steps of the test, which consisted of five substeps, the participants were asked to place as many pins as possible into the holes with the right hand (step one), left hand (step two), and both hands (step three) for 30 seconds. The score of each step at the end of the time was recorded. In the fourth step, the sum of the first three evaluation results was calculated. Finally, in the assembly step, the participants put pins, washers, and collars on top by using both hands together within 60 seconds. The score of the fifth step was obtained by multiplying the number of pins, washers, and collars placed within 60 seconds by 4 [19]. Dual-task performance was evaluated by adding the cognitive and motor task performance during the PPT. According to the educational status of the participants, their cognitive task was to subtract a series of threes and sevens starting from a randomly selected number between 290 and 310, counting names/cities starting with a specific letter, or counting down the days of the week starting from a randomly selected day. The participants were asked to tap their foot with the less affected side (PD group) or the nondominant side (control group) as a motor task. Scores were calculated as for the PPT.
- Motor speed was assessed using the finger tapping test (FTT). Participants were asked to place the forearm on the table in the pronated position while sitting and to touch a designated area as quickly as possible using their index fingers. The number of taps in 60 seconds was recorded [20].
- The participants’ hand grip strength (HGS) was evaluated with a hand dynamometer (Saehan SH5001 Hydraulic Hand Dynamometer, Saehan Corporation, Masan, Korea), and pinch strength was evaluated with a pinch meter (Saehan SH5005 Hydraulic Pinch Gauge, Saehan Corporation, Masan, Korea). HGS measurement was performed as recommended by the American Association of Hand Therapists. In the sitting position, with the shoulder in adduction, the elbow at 90° flexion, and the forearm in the neutral position, the participants did three repetitions with the right side and three with the left side at one-minute intervals, and the averages were recorded. Pinch force was measured at one-minute intervals, with three repetitions in three standard positions: the palmar grip, tip grip, and lateral grip. The averages were recorded [21].
- Statistical analysis
- Statistical analysis was conducted using IBM SPSS 23.0 software (IBM Corp., Armonk, NY, USA). Data are presented as percentages or means with standard deviations. Baseline descriptive statistics are reported, and the Shapiro‒Wilk test was used to test for normality. Qualitative variables were compared between groups by the chi-square test. The analysis of covariance (ANCOVA) was conducted to compare cervical JPE, cervical muscle strength and endurance, manual dexterity, and hand strength differences between groups, and variables were adjusted for age, sex and disease duration. The Mann‒Whitney U test was conducted to compare PD patients with different posture scores. Pearson’s partial correlation was run to assess the relationship between cervical JPE and muscle strength and endurance with manual dexterity and hand strength after controlling for age, disease duration, action tremor, resting tremor, bradykinesia and rigidity factors in patients with PD. Pearson’s correlation test was used to assess the relationship of cervical JPE, cervical muscle strength and endurance with manual dexterity and hand strength among healthy controls. We estimated that a sample size of 20 for each group would have a 95% confidence level and 90% power to detect a significant difference in manual dexterity score between Parkinson’s and healthy individuals, which indicates an effect size of 1.13 [22].
MATERIALS & METHODS
Unified Parkinson’s Disease Rating Scale
Joint position error
Craniocervical Flexion Test
Neck flexor muscle endurance test
Neck extensor muscle endurance test
Purdue Pegboard Test and dual task
Finger tapping test
Grip strength and pinch strength
- The demographic and clinical characteristics of the participants are given in Table 1. There was no significant difference between the PD and control groups regarding age, sex, height, or weight.
- When the PD and control groups were compared, the endurance of the cervical flexor and extensor muscles (p = 0.017, p < 0.001) and CCFT result (performance index: p = 0.001) were significantly lower in the PD group. Cervical JPE (flexion: p = 0.039, right rotation: p = 0.018, left rotation: p = 0.019) was significantly higher in the PD group. PPT (p < 0.001), the cognitive task of the PPT (p < 0.001), the motor task of the PPT (p < 0.001), and the FTT score (dominant: p = 0.015, nondominant p = 0.036) were significantly lower in the PD group. HGS (dominant: p = 0.020, nondominant: p = 0.003) and tip pinch strength (dominant: p = 0.039, nondominant: p = 0.001) were significantly lower in the PD group for both hands. There was no significant difference between the groups regarding palmar or lateral pinch strength (p > 0.05) (Table 2).
- Comparing participants with a UPDRS posture score of 1 and > 1, the cervical extensor endurance was significantly lower in those with a posture score of > 1 (p = 0.005) (Table 3). In the PD group, cervical flexion JPE with PPT (less affected hand: r = -0.590, p = 0.026; both hands: r = -0.592, p = 0.026; total: r = -0.626, p = 0.017), the cognitive task of the PPT (both hands: r = -0.668, p = 0.009) and motor task of the PPT (both hands: r = -0.601, p = 0.023; total: r = -0.557, p = 0.038; assembly: r = -0.597, p = 0.024) measurement results were negatively correlated (p < 0.05) (Table 4). While there was a positive correlation of cervical flexor endurance with HGS (most affected hand: r = 0.584, p = 0.028, less affected hand: r = 0.692, p = 0.006), tip pinch strength (most affected hand: r = 0.538, p = 0.047) and palmar pinch strength (most affected hand: r = 0.649, p = 0.012, less affected hand: r = 0.556, p = 0.039), a negative correlation was found with PPT (most affected hand: r = -0.543, p = 0.045; both hands: r = -0.550, p = 0.041; total: r = -0.561, p = 0.037; assembly: r = -0.684, p = 0.007) and cognitive task of the PPT (most affected hand: r = -0.667, p = 0.009; both hands: r = -0.619, p = 0.018; total: r = -0.603, p = 0.022). There was a negative correlation between the endurance of cervical extensors and the measurement results of the cognitive task of the PPT (less affected hand: r = -0.699, p = 0.005). In addition, there was a negative correlation between the CCFT performance index and PPT (assembly: r = -0.577, p = 0.031) measurement results (Table 4).
- Cervical extension JPE was negatively correlated with PPT score (dominant hand: r = -0.447, p = 0.048; assembly: r = -0.541, p = 0.014), the cognitive task of the PPT (both hands: r = -0.469, p = 0.037; total: r = -0.445, p = 0.049; assembly: r = -0.511, p = 0.021) and the motor task of the PPT (dominant hand: r = -0.459, p = 0.042; nondominant hand: r = -0.624, p = 0.003; both hands: r = -0.703, p = 0.001; total: r = -0.630, p = 0.003; assembly: r = -0.478, p = 0.033) in the healthy individuals. The cognitive task of the PPT was positively correlated with cervical extensor endurance, CCFT activation score, and CCFT performance index (p < 0.05). There was a positive correlation of cervical flexor endurance, cervical extensor endurance, and CCFT performance index with HGS measurements (p < 0.05). There was a positive correlation of cervical extensor endurance, CCFT activation score and CCFT performance index with pinch strength measurements (p < 0.05) (Table 5).
RESULTS
- This cross-sectional study investigated the effects of cervical proprioception and cervical muscle strength and endurance on manual dexterity and hand strength in individuals with PD. Compared to healthy individuals, cervical proprioception and cervical muscle strength and endurance were significantly decreased in individuals with PD. Good performance on manual dexterity tests in the PD group was associated with cervical proprioception. Cervical extensor endurance was significantly lower in individuals with PD in those with more postural deformity. In addition, increased cervical flexor endurance was associated with increased HGS, tip pinch strength, and palmar pinch strength. As an interesting result of this study, a negative correlation was found between neck flexor muscle endurance and manual dexterity test results. This result may have emerged due to the compensation mechanisms developed by Parkinson’s patients in multijoint movements.
- In this study, cervical JPE was considerably higher in individuals with PD than in healthy individuals. In a previous study examining age-related cervical JPE in healthy individuals, it was reported that the average degree of cervical JPE was 5° for flexion and 6° for extension and right-left rotation in individuals over age 50 [23]. While the JPE values of the healthy controls in our study were similar to this, the values were approximately two times higher in individuals with PD. Although there are studies [23-27] investigating cervical proprioception in various groups, as far as we know, this is the first study to investigate the impact of cervical proprioception on individuals with PD. Studies have also specified that cervical proprioception is affected by postural changes [24-26]. Ha and Sung [24] reported that a temporary forward head posture develops during 40 minutes of smartphone use in healthy individuals affects cervical proprioception negatively. Lee et al. [25] showed that cervical proprioception decreases in individuals with a forward head posture, and cervical proprioception worsens as this posture becomes more severe. Alghadir et al. [26] also examined the effect of different sitting positions on cervical proprioception in healthy individuals and demonstrated that cervical proprioception changes depending on body posture. A flexion posture typically develops due to reasons such as rigidity, dystonia, impaired postural control, and soft tissue changes in individuals with PD, and more severe postural deformities such as camptocormia, antecollis, Pisa syndrome, and scoliosis may also be seen in some patients [28]. Alwardat et al. [29] reported that neck disability index scores were higher in PD patients with postural deformities such as Pisa syndrome and camptocarmia. Forsyth et al. [28] showed that increased flexion posture and decreased thoracic proprioception were associated with mild to moderate PD. In the present study, the UPDRS score on the posture item was 1 in 70%, 2 in 20%, and 3 in 10%. These scores indicate that there are postural changes in individuals with PD, most of whom are at an early stage according to the Hoehn Yahr Staging. We think that the decrease in cervical proprioception in the PD group compared to the control group may be related to the postural changes typically seen in PD. When we compared the participants with different UPDRS posture scores, cervical extensor endurance was significantly lower in those with more postural deformity. Cervical muscle spindles are the main proprioceptors of the neck and are particularly abundant in the suboccipital muscles. Changes in the functioning of the cervical muscles affect cervical proprioception by altering the discharge of muscle spindles [9]. It has been reported that a decrease in the endurance of cervical extensors in healthy individuals increases the cervical JPE [30]. Therefore, we think that as the severity of postural deformities progresses, proprioception may worsen along with cervical extensor endurance.
- Manual dexterity, HGS and tip grip strength were decreased in individuals with PD in this study. Various studies have shown that manual dexterity decreases in individuals with PD compared to healthy individuals [31,32]. Isometric finger torque production and control are affected in PD [3]. In addition, bradykinesia, tremor, rigidity, difficulty in sequential movements, and impaired ability to synchronize and coordinate movements affect manual dexterity [2-4,33]. While the integration of proprioceptive signals, especially visual information, has an important role in guiding and correcting hand movements, the integration and feedback of sensorimotor information into an intended motor output may also affect hand skills in PD [33]. Studies have also reported that people with mild to moderate PD have lower grip strength than healthy controls [31,32]. The mechanism by which grip strength decreases in PD is not clear. It has been reported that corticospinal activation of the muscle may be impaired due to the decrease in nigro-striatal dopamine. This impairment may lead to muscle weakness by altering motor unit uptake [34]. It has also been associated with a decrease in torque production rate [31].
- In this study, poor upper extremity performance in the PD group was associated with decreased cervical proprioception. In healthy individuals, the decrease in cervical proprioception was associated with lower upper extremity performance, while the increase in the strength of the deep cervical muscles was associated with higher upper extremity performance. Studies have shown a relationship between posture and upper extremity performance in healthy individuals and various diseases, including PD [5,28,35,36]. Kwon et al. [12] reported that upper extremity muscle activity was impaired by poor the head posture of healthy individuals with forward head posture and rounded shoulder posture. Gillen et al. [35] examined upper extremity function under different trunk postures in healthy individuals and reported that the neutral position of the trunk might increase upper extremity performance. Kalkan et al. [5] investigated postural control and upper extremity dexterity and showed a relationship between postural control and manual dexterity in individuals with PD over 65 years old. Forsyth et al. [28] reported that flexion posture negatively affects upper extremity activity in individuals with mild and moderate PD. Alwardat et al. [36] reported that Pisa syndrome is associated with major impairments in upper extremity function and activities of daily living in PD.
- The cervical spine contains proprioceptors that sense the position and movement of neck muscles, ligaments, and joints. The cerebellum integrates sensory feedback from the cervical region with other sensory inputs, such as visual and vestibular information, to modulate and improve motor commands [7,8]. Cervical input from the spinocerebellar tracts is matched against the body schema to predict the future position of each limb [14] and assists the cerebellum in regulating and coordinating movements involving the neck, head, and upper extremities. It also contributes to the control of balance, postural control, head position and fine motor skills in tasks that require precise movements of the neck and upper body [9,10,37,38]. Therefore, changes in head and neck position may affect the perceived position of body parts relative to each other, the perception of the target position, and/or the perception of body position by causing changes in proprioceptive afferent information [13]. Knox and Hodges [13] showed that changes in head and neck position in the absence of visual cues in healthy individuals affect the processing of afferent sensory inputs and may alter elbow proprioception. Ünlüer et al. [11] reported that shoulder proprioception and upper extremity function decreased due to neck pain in individuals over 65 years old. In our study, the alteration of afferent information from the cervical region due to impaired cervical proprioception in the PD group may have affected the inner body schema and decreased upper extremity proprioception. As a result, it is possible that goal-oriented upper extremity skills in particular have decreased. In a systematic review conducted by Pennington et al. [39], executive dysfunction, attention deficit, and visuospatial difficulties were found to be associated with cognitive involvement in PD. Upper extremity performance may have been adversely affected by the distraction during goal-oriented movements and the inability to obtain correct information from the body schema, which creates a need for more internal input. This may also explain the further decrease in upper extremity performance, especially during cognitive dual tasks. In addition, there are abnormalities in sensorimotor integration that cause motor problems in PD. Somatosensory abnormalities are present in PD, including decreased proprioceptive function, impaired haptic and tactile perception, caused errors in spatiotemporal discriminative sense, and altered mechanical pain perception [40,41]. It has been reported that such proprioceptive abnormalities may be associated with changes in the cortical processing of kinesthetic signals and may also be the source of scaling problems in voluntary movements [42]. Impaired posture and dexterity due to these sensory abnormalities may also be a possible explanation.
- Visual and muscle afferents are essential for limb proprioception during limb movement, as visual and kinesthetic inputs must be continuously matched to predict future limb positions. Muscle spindles, especially dense in the deep cervical muscles, work as the main proprioceptors [10]. Deep cervical flexors provide stability and maintain the neck’s neutral posture during fixed posture or prolonged activity [6]. To the best of our knowledge, no study has examined the relationship of the strength and endurance of the cervical muscles with manual dexterity and hand strength in PD. Abdelkader et al. [27] specified that fatigue of the cervical flexor muscles in healthy individuals reduces cervical proprioception and postural stability. Radosher et al. [43] indicated that deep cervical flexor cross-sectional area and density were negatively associated with upper extremity pain and disability in individuals with neck pain. Studies have also reported in healthy individuals that cervical extensor muscle fatigue may affect upper extremity kinesthesia and change the hand-eye tracking path due to impaired upper extremity proprioception in the absence of visual guidance [10,14]. Reece et al. [44] stated that wrist proprioception was affected in individuals with neck pain and cervical extensor muscle fatigue. In this study, we hypothesized that the deterioration of the cervical neutral posture due to the decrease in the endurance of the cervical muscles in the PD group and the changes in the proprioceptive functioning of the cervical muscles would affect the upper extremity negatively by changing the body schema. As we expected, the strength and endurance of the cervical muscles in individuals with PD were significantly lower than those in healthy individuals, and an increase in cervical flexor endurance was associated with increased HGS and tip and palmar pinch strength. Cervical extensor endurance was decreased in individuals with PD who had more postural deformity. Similarly, the increase in the strength and endurance of the cervical muscles was associated with increased HGS and pinch strength in healthy individuals. At the same time, surprisingly, there was a negative correlation between the endurance of the cervical flexors and manual dexterity. Based on our clinical experience, we think that this negative correlation may be related to the change in movement control and the development of compensation mechanisms due to the disease when PD patients engage in upper extremity tasks in which more than one joint is used, independent of the decrease in the endurance of the cervical flexors. A few studies support this view [45,46]. To explain the mechanisms affecting manual dexterity in PD, it would be useful to examine the response or activity of cervical muscles during upper extremity tasks with methods such as electromyography.
- The current study had several limitations. First, upper extremity proprioception was not evaluated. Evaluation of upper extremity proprioception could also be useful to support the results of the present study, but including this measurement can be tired the individuals with PD. The evaluation of upper extremity proprioception may be considered in future studies. Second, we did not adjust our threshold of significance (p < 0.05) for multiple-hypothesis testing. We believe that this was justified given the exploratory nature of our study, which tests hypotheses concerning possible relationships of cervical proprioception, cervical muscle strength, and endurance with manual dexterity and hand strength in individuals with PD.
- In conclusion, cervical region proprioception, the strength and endurance of cervical muscles, manual dexterity, and hand strength decrease in PD patients compared to healthy individuals. Impairment of cervical proprioception appears to be associated with poorer upper extremity performance. In treating individuals with early- and middle-stage PD, approaches to preventing postural deformities and improving normal posture may effectively reduce upper extremity problems. In particular, approaches to improve cervical region proprioception can help patients increase their upper extremity function and perform activities that require dexterity in daily life. The findings of this study support the importance of detailed evaluation of the cervical region in PD and may lead to the development of cervical region-focused rehabilitation programs.
DISCUSSION
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Funding Statement
None
-
Author contributions
Conceptualization: all authors. Data curation: Özlem Menevşe, Büşra Kepenek-Varol. Formal analysis: Büşra Kepenek-Varol. Methodology: Özlem Menevşe, Büşra Kepenek-Varol, Murat Gültekin, Sevil Bilgin. Resources: Murat Gültekin. Writing—original draft: Özlem Menevşe. Writing—review & editing: Sevil Bilgin, Büşra Kepenek-Varol.
Notes
- The authors express gratitude to Aynur Demirel PT, PhD, Assoc. Prof. (Faculty of Physical Therapy and Rehabilitation, Hacettepe University, Ankara, Turkey) and Halil İbrahim Çelik (Division of Statistics, Department of Data Science, Faculty of Sciences, Gazi University, Ankara, Turkey) for their statistical support.
Acknowledgments
| PD (n = 20) | Healthy controls (n = 20) | p value | |||
|---|---|---|---|---|---|
| Cervical JPE | |||||
| Flexion | 10.1 ± 3.89 | 5.33 ± 3.13 | 0.039* | ||
| Extension | 12.5 ± 5.71 | 7.99 ± 4.54 | 0.129 | ||
| Right rotation | 13.2 ± 6.47 | 6.03 ± 3.17 | 0.018* | ||
| Left rotation | 14.5 ± 8.97 | 6.04 ± 3.72 | 0.019* | ||
| Cervical muscle strength and endurance tests | |||||
| CCFT activation score | 0.9 ± 1.02 | 2 ± 1.59 | 0.148 | ||
| CCFT performance index | 8.7 ± 5.93 | 25.2 ± 12.5 | 0.001* | ||
| Flexor endurance | 23.8 ± 13.9 | 44.9 ± 22.7 | 0.017* | ||
| Extensor endurance | 58.3 ± 38.1 | 223.7 ± 84.1 | < 0.001* | ||
| Manuel dexterity | |||||
| PPT | |||||
| Dominant hand | 8.85 ± 2.7 | 14.3 ± 1.97 | < 0.001* | ||
| Non-dominant hand | 8.7 ± 2.27 | 13.5 ± 2.14 | < 0.001* | ||
| Both hands | 6 ± 2.1 | 10.3 ± 1.39 | < 0.001* | ||
| Total | 23.5 ± 6.41 | 38 ± 4.72 | < 0.001* | ||
| Assembly task | 13 ± 4.08 | 24.4 ± 6.07 | < 0.001* | ||
| Cognitive tasks of the PPT | |||||
| Dominant hand | 6.65 ± 2.74 | 12.5 ± 1.93 | < 0.001* | ||
| Non-dominant hand | 6.5 ± 2.48 | 11.7 ± 2.39 | < 0.001* | ||
| Both hands | 5.15 ± 2.13 | 9.5 ± 1.76 | < 0.001* | ||
| Total | 18.3 ± 6.99 | 33.6 ± 5.72 | < 0.001* | ||
| Assembly task | 8 ± 3.67 | 16.8 ± 5.89 | < 0.001* | ||
| Motor tasks of the PPT | |||||
| Dominant hand | 9 ± 2.64 | 14.3 ± 2.31 | < 0.001* | ||
| Non-dominant hand | 8.4 ± 2.66 | 13.1 ± 2.35 | < 0.001* | ||
| Both hands | 5.25 ± 2.45 | 10.6 ± 2.16 | < 0.001* | ||
| Total | 22.6 ± 7.12 | 37.9 ± 6.42 | < 0.001* | ||
| Assembly task | 11.8 ± 5.73 | 24 ± 7.11 | < 0.001* | ||
| FTT | |||||
| Dominant hand | 137 ± 28.7 | 169 ± 33.4 | 0.015* | ||
| Non-dominant hand | 136 ± 31.4 | 164 ± 34.4 | 0.036* | ||
| Hand strength | |||||
| Grip strength, kg | |||||
| Dominant hand | 26.8 ± 9.5 | 33.1 ± 9.65 | 0.020* | ||
| Non-dominant hand | 24.2 ± 8.93 | 31.1 ± 9.66 | 0.003* | ||
| Tip pinch, kg | |||||
| Dominant hand | 5.79 ± 1.49 | 7.51 ± 2.45 | 0.039* | ||
| Non-dominant hand | 5.41 ± 1.13 | 6.99 ± 1.72 | 0.001* | ||
| Palmar pinch, kg | |||||
| Dominant hand | 6.85 ± 1.8 | 7.88 ± 1.84 | 0.151 | ||
| Non-dominant hand | 6.48 ± 1.85 | 7.23 ± 1.68 | 0.073 | ||
| Lateral pinch, kg | |||||
| Dominant hand | 8.84 ± 2.48 | 9.44 ± 2.37 | 0.887 | ||
| Non-dominant hand | 8.45 ± 2.38 | 8.95 ± 2.37 | 0.509 | ||
Data are presented as mean ± standard deviation. p value was adjusted for age, sex and disease duration using analysis of covariance (ANCOVA).
* p value < 0.05.
JPE, joint position error; PD, Parkinson’s disease; CCFT, Craniocervical Flexion Test; PPT, Purdue Pegboard Test; FTT, finger tapping test.
|
UPDRS posture |
p value | ||
|---|---|---|---|
| Score = 1 (n = 14) | Score > 1 (n = 6) | ||
| Cervical JPE | |||
| Flexion | 10.1 ± 2.51 | 10.3 ± 6.42 | 0.508 |
| Extension | 12.1 ± 6.24 | 13.4 ± 4.61 | 0.386 |
| Right rotation | 12.5 ± 4.24 | 14.7 ± 10.4 | 0.869 |
| Left rotation | 14.2 ± 7.85 | 15.2 ± 12 | 0.680 |
| Cervical muscle strength and endurance tests | |||
| CCFT activation score | 0.71 ± 0.99 | 1.33 ± 1.03 | 0.214 |
| CCFT performance index | 8.43 ± 6.14 | 9.33 ± 5.89 | 0.501 |
| Flexor endurance | 24.5 ± 13.9 | 22.3 ± 15.3 | 0.650 |
| Extensor endurance | 72.2 ± 36.8 | 25.8 ± 13.6 | 0.005* |
|
Cervical JPE flexion |
Cervical JPE extension |
Cervical JPE right rotation |
Cervical JPE left rotation |
Cervical flexor endurance |
Cervical extensor endurance |
CCFT activation score |
CCFT performance index |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | ||
| PPT | |||||||||||||||||
| Most affected hand | -0.390 | 0.168 | -0.031 | 0.917 | 0.043 | 0.883 | 0.105 | 0.720 | -0.543 | 0.045* | -0.234 | 0.421 | -0.371 | 0.192 | -0.299 | 0.299 | |
| Less affected hand | -0.590 | 0.026* | -0.211 | 0.469 | -0.245 | 0.399 | -0.177 | 0.545 | -0.367 | 0.196 | -0.470 | 0.090 | 0.065 | 0.824 | -0.124 | 0.673 | |
| Both hands | -0.592 | 0.026* | -0.357 | 0.211 | -0.117 | 0.690 | -0.022 | 0.940 | -0.550 | 0.041* | -0.058 | 0.843 | 0.075 | 0.798 | -0.079 | 0.787 | |
| Total | -0.626 | 0.017* | -0.246 | 0.398 | -0.141 | 0.630 | -0.053 | 0.857 | -0.561 | 0.037* | -0.309 | 0.282 | -0.063 | 0.830 | -0.185 | 0.526 | |
| Assembly task | -0.449 | 0.107 | -0.245 | 0.399 | -0.078 | 0.791 | -0.296 | 0.305 | -0.684 | 0.007* | 0.083 | 0.779 | -0.332 | 0.246 | -0.577 | 0.031* | |
| Cognitive tasks of the PPT | |||||||||||||||||
| Most affected hand | -0.461 | 0.097 | 0.002 | 0.995 | 0.297 | 0.302 | 0.072 | 0.808 | -0.667 | 0.009* | -0.293 | 0.310 | 0.035 | 0.906 | -0.136 | 0.644 | |
| Less affected hand | -0.221 | 0.448 | 0.177 | 0.545 | 0.233 | 0.424 | 0.168 | 0.565 | -0.404 | 0.152 | -0.699 | 0.005* | 0.206 | 0.480 | 0.149 | 0.611 | |
| Both hands | -0.668 | 0.009* | 0.014 | 0.961 | -0.010 | 0.974 | -0.055 | 0.852 | -0.619 | 0.018* | -0.455 | 0.102 | 0.127 | 0.665 | -0.133 | 0.650 | |
| Total | -0.468 | 0.092 | 0.073 | 0.804 | 0.202 | 0.489 | 0.076 | 0.797 | -0.603 | 0.022* | -0.521 | 0.056 | 0.131 | 0.654 | -0.036 | 0.902 | |
| Assembly task | -0.500 | 0.068 | -0.264 | 0.361 | 0.264 | 0.361 | 0.173 | 0.555 | -0.278 | 0.336 | -0.085 | 0.772 | 0.177 | 0.545 | -0.086 | 0.770 | |
| Motor tasks of the PPT | |||||||||||||||||
| Most affected hand | -0.407 | 0.149 | -0.147 | 0.616 | 0.044 | 0.882 | 0.187 | 0.521 | -0.321 | 0.263 | -0.050 | 0.864 | 0.036 | 0.904 | 0.108 | 0.714 | |
| Less affected hand | -0.456 | 0.101 | -0.052 | 0.859 | 0.222 | 0.446 | 0.172 | 0.557 | -0.435 | 0.120 | -0.333 | 0.245 | -0.029 | 0.921 | -0.239 | 0.410 | |
| Both hands | -0.601 | 0.023* | -0.346 | 0.225 | 0.181 | 0.536 | 0.160 | 0.585 | -0.353 | 0.216 | -0.062 | 0.834 | 0.185 | 0.527 | -0.042 | 0.886 | |
| Total | -0.557 | 0.038* | -0.208 | 0.475 | 0.167 | 0.568 | 0.199 | 0.495 | -0.422 | 0.133 | -0.166 | 0.570 | 0.073 | 0.805 | -0.061 | 0.837 | |
| Assembly task | -0.597 | 0.024* | -0.456 | 0.101 | -0.144 | 0.624 | -0.090 | 0.760 | -0.526 | 0.053 | 0.219 | 0.452 | -0.078 | 0.790 | -0.223 | 0.444 | |
| FTT | |||||||||||||||||
| Most affected hand | 0.015 | 0.959 | -0.117 | 0.692 | -0.135 | 0.645 | -0.298 | 0.301 | 0.402 | 0.154 | 0.111 | 0.705 | 0.213 | 0.464 | 0.096 | 0.745 | |
| Less affected hand | -0.029 | 0.922 | -0.262 | 0.365 | -0.215 | 0.460 | -0.141 | 0.630 | 0.426 | 0.129 | 0.015 | 0.960 | 0.125 | 0.671 | 0.114 | 0.698 | |
| Grip strength, kg | |||||||||||||||||
| Most affected hand | 0.148 | 0.615 | -0.136 | 0.643 | 0.246 | 0.397 | 0.234 | 0.420 | 0.584 | 0.028* | 0.399 | 0.158 | 0.310 | 0.280 | -0.048 | 0.870 | |
| Less affected hand | 0.242 | 0.404 | 0.151 | 0.606 | 0.366 | 0.198 | 0.256 | 0.378 | 0.692 | 0.006* | 0.289 | 0.317 | 0.331 | 0.248 | -0.118 | 0.689 | |
| Tip pinch, kg | |||||||||||||||||
| Most affected hand | 0.295 | 0.306 | -0.080 | 0.785 | 0.239 | 0.411 | 0.272 | 0.347 | 0.538 | 0.047* | 0.356 | 0.212 | 0.520 | 0.057 | -0.055 | 0.852 | |
| Less affected hand | 0.081 | 0.782 | 0.017 | 0.955 | 0.130 | 0.659 | 0.110 | 0.708 | 0.293 | 0.309 | 0.487 | 0.078 | 0.217 | 0.456 | -0.323 | 0.260 | |
| Palmar pinch, kg | |||||||||||||||||
| Most affected hand | 0.447 | 0.109 | 0.126 | 0.668 | 0.349 | 0.221 | 0.473 | 0.087 | 0.649 | 0.012* | 0.320 | 0.265 | 0.348 | 0.223 | -0.041 | 0.890 | |
| Less affected hand | 0.320 | 0.265 | 0.088 | 0.764 | 0.277 | 0.337 | 0.329 | 0.251 | 0.556 | 0.039* | 0.413 | 0.142 | 0.508 | 0.063 | -0.195 | 0.505 | |
| Lateral pinch, kg | |||||||||||||||||
| Most affected hand | 0.323 | 0.260 | -0.150 | 0.608 | 0.357 | 0.211 | 0.401 | 0.156 | 0.526 | 0.053 | 0.470 | 0.090 | 0.508 | 0.063 | 0.019 | 0.949 | |
| Less affected hand | 0.229 | 0.430 | -0.147 | 0.615 | 0.292 | 0.311 | 0.341 | 0.233 | 0.486 | 0.078 | 0.431 | 0.124 | 0.466 | 0.093 | -0.041 | 0.890 | |
|
Cervical JPE flexion |
Cervical JPE extension |
Cervical JPE right rotation |
Cervical JPE left rotation |
Cervical flexor endurance |
Cervical extensor endurance |
CCFT activation score |
CCFT performance index |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | ||
| PPT | |||||||||||||||||
| Dominant hand | 0.276 | 0.239 | -0.447 | 0.048* | 0.164 | 0.488 | 0.102 | 0.670 | 0.013 | 0.957 | 0.345 | 0.136 | 0.336 | 0.147 | 0.277 | 0.237 | |
| Non-dominant hand | -0.149 | 0.530 | -0.233 | 0.324 | -0.119 | 0.617 | -0.048 | 0.840 | 0.043 | 0.856 | 0.231 | 0.326 | 0.062 | 0.795 | 0.081 | 0.735 | |
| Both hands | -0.073 | 0.760 | -0.433 | 0.051 | -0.083 | 0.729 | -0.064 | 0.787 | 0.232 | 0.324 | 0.236 | 0.317 | 0.143 | 0.547 | 0.114 | 0.633 | |
| Total | 0.040 | 0.866 | -0.418 | 0.066 | -0.002 | 0.992 | 0.058 | 0.807 | 0.092 | 0.700 | 0.317 | 0.173 | 0.210 | 0.374 | 0.183 | 0.440 | |
| Assembly task | 0.181 | 0.446 | -0.541 | 0.014* | -0.001 | 0.997 | -0.085 | 0.723 | 0.048 | 0.841 | 0.461 | 0.041* | 0.174 | 0.462 | 0.242 | 0.304 | |
| Cognitive tasks of the PPT | |||||||||||||||||
| Dominant hand | 0.275 | 0.240 | -0.355 | 0.124 | 0.255 | 0.277 | 0.219 | 0.353 | 0.127 | 0.593 | 0.109 | 0.648 | 0.514 | 0.020* | 0.402 | 0.079 | |
| Non-dominant hand | 0.134 | 0.574 | -0.433 | 0.056 | 0.120 | 0.615 | 0.000 | > 0.999 | 0.056 | 0.813 | 0.236 | 0.316 | 0.416 | 0.068 | 0.442 | 0.051 | |
| Both hands | 0.190 | 0.421 | -0.469 | 0.037* | 0.063 | 0.792 | 0.044 | 0.853 | 0.285 | 0.223 | 0.291 | 0.214 | 0.526 | 0.017* | 0.486 | 0.030* | |
| Total | 0.207 | 0.381 | -0.445 | 0.049* | 0.155 | 0.513 | 0.088 | 0.713 | 0.154 | 0.516 | 0.225 | 0.341 | 0.509 | 0.022* | 0.470 | 0.037* | |
| Assembly task | -0.061 | 0.798 | -0.511 | 0.021* | -0.065 | 0.785 | -0.053 | 0.825 | 0.058 | 0.808 | 0.531 | 0.016* | 0.225 | 0.340 | 0.328 | 0.157 | |
| Motor tasks of the PPT | |||||||||||||||||
| Dominant hand | 0.172 | 0.469 | -0.459 | 0.042* | 0.260 | 0.269 | 0.154 | 0.518 | -0.084 | 0.724 | 0.169 | 0.477 | 0.286 | 0.221 | 0.221 | 0.348 | |
| Non-dominant hand | 0.140 | 0.555 | -0.624 | 0.003* | 0.155 | 0.514 | -0.062 | 0.794 | 0.040 | 0.868 | 0.361 | 0.118 | 0.254 | 0.281 | 0.294 | 0.208 | |
| Both hands | 0.215 | 0.363 | -0.703 | 0.001† | 0.120 | 0.615 | 0.106 | 0.658 | 0.158 | 0.507 | 0.372 | 0.106 | 0.429 | 0.059 | 0.415 | 0.069 | |
| Total | 0.186 | 0.434 | -0.630 | 0.003† | 0.191 | 0.421 | 0.068 | 0.775 | 0.037 | 0.876 | 0.318 | 0.172 | 0.340 | 0.142 | 0.327 | 0.159 | |
| Assembly task | 0.416 | 0.068 | -0.478 | 0.033* | 0.165 | 0.486 | 0.088 | 0.712 | 0.050 | 0.836 | 0.414 | 0.070 | 0.298 | 0.202 | 0.250 | 0.287 | |
| FTT | |||||||||||||||||
| Dominant hand | 0.064 | 0.787 | -0.155 | 0.515 | -0.035 | 0.884 | -0.340 | 0.143 | 0.234 | 0.321 | 0.291 | 0.213 | -0.079 | 0.740 | -0.126 | 0.598 | |
| Non-dominant hand | 0.033 | 0.889 | -0.198 | 0.404 | -0.403 | 0.078 | -0.407 | 0.075 | 0.216 | 0.360 | 0.338 | 0.145 | -0.056 | 0.815 | -0.143 | 0.548 | |
| Grip strength, kg | |||||||||||||||||
| Dominant hand | -0.237 | 0.315 | -0.238 | 0.313 | -0.112 | 0.640 | -0.251 | 0.285 | 0.445 | 0.049* | 0.522 | 0.018* | 0.352 | 0.128 | 0.588 | 0.006† | |
| Non-dominant hand | -0.208 | 0.379 | -0.230 | 0.329 | -0.108 | 0.649 | -0.216 | 0.361 | 0.411 | 0.072 | 0.549 | 0.012* | 0.370 | 0.108 | 0.606 | 0.005† | |
| Tip pinch, kg | |||||||||||||||||
| Dominant hand | -0.243 | 0.303 | -0.112 | 0.638 | -0.051 | 0.830 | -0.192 | 0.417 | 0.253 | 0.281 | 0.330 | 0.156 | 0.221 | 0.349 | 0.433 | 0.057 | |
| Non-dominant hand | -0.139 | 0.560 | -0.110 | 0.643 | 0.065 | 0.784 | -0.085 | 0.722 | 0.267 | 0.256 | 0.354 | 0.126 | 0.298 | 0.203 | 0.478 | 0.033* | |
| Palmar pinch, kg | |||||||||||||||||
| Dominant hand | -0.075 | 0.755 | -0.345 | 0.136 | -0.049 | 0.839 | -0.065 | 0.785 | 0.437 | 0.054 | 0.553 | 0.011* | 0.412 | 0.071 | 0.628 | 0.003† | |
| Non-dominant hand | -0.237 | 0.315 | -0.241 | 0.305 | -0.053 | 0.823 | -0.039 | 0.869 | 0.294 | 0.209 | 0.348 | 0.133 | 0.321 | 0.167 | 0.529 | 0.016* | |
| Lateral pinch, kg | |||||||||||||||||
| Dominant hand | -0.006 | 0.979 | -0.306 | 0.190 | -0.090 | 0.705 | -0.115 | 0.630 | 0.364 | 0.115 | 0.561 | 0.010* | 0.442 | 0.051 | 0.680 | 0.001† | |
| Non-dominant hand | 0.019 | 0.937 | -0.258 | 0.272 | -0.091 | 0.701 | -0.030 | 0.899 | 0.265 | 0.258 | 0.524 | 0.018* | 0.538 | 0.014* | 0.746 | < 0.001† | |
- 1. Hayes MT. Parkinson’s disease and Parkinsonism. Am J Med 2019;132:802–807.ArticlePubMed
- 2. Quinn L, Busse M, Dal Bello-Haas V. Management of upper extremity dysfunction in people with Parkinson disease and Huntington disease: facilitating outcomes across the disease lifespan. J Hand Ther 2013;26:148–154.quiz 155. ArticlePubMed
- 3. Oliveira MA, Rodrigues AM, Caballero RM, Petersen RD, Shim JK. Strength and isometric torque control in individuals with Parkinson’s disease. Exp Brain Res 2008;184:445–450.ArticlePubMedPDF
- 4. Lee KS, Lee WH, Hwang S. Modified constraint-induced movement therapy improves fine and gross motor performance of the upper limb in Parkinson disease. Am J Phys Med Rehabil 2011;90:380–386.ArticlePubMed
- 5. Kalkan AC, Kahraman T, Ugut BO, Colakoglu BD, Genc A. A comparison of the relationship between manual dexterity and postural control in young and older individuals with Parkinson’s disease. J Clin Neurosci 2020;75:89–93.ArticlePubMed
- 6. Amiri Arimi S, Ghamkhar L, Kahlaee AH. The relevance of proprioception to chronic neck pain: a correlational analysis of flexor muscle size and endurance, clinical neck pain characteristics, and proprioception. Pain Med 2018;19:2077–2088.ArticlePubMed
- 7. Röijezon U, Clark NC, Treleaven J. Proprioception in musculoskeletal rehabilitation. Part 1: basic science and principles of assessment and clinical interventions. Man Ther 2015;20:368–377.ArticlePubMed
- 8. de Vries J, Ischebeck BK, Voogt LP, van der Geest JN, Janssen M, Frens MA, et al. Joint position sense error in people with neck pain: a systematic review. Man Ther 2015;20:736–744.ArticlePubMed
- 9. Peng B, Yang L, Li Y, Liu T, Liu Y. Cervical proprioception impairment in neck pain-pathophysiology, clinical evaluation, and management: a narrative review. Pain Ther 2021;10:143–164.ArticlePubMedPMCPDF
- 10. Zabihhosseinian M, Holmes MW, Murphy B. Neck muscle fatigue alters upper limb proprioception. Exp Brain Res 2015;233:1663–1675.ArticlePubMedPDF
- 11. Ünlüer NÖ, Ateş Y. An investigation of neck pain in older adults, and its relation with shoulder position sense and upper extremity function. Somatosens Mot Res 2021;38:333–338.ArticlePubMed
- 12. Kwon JW, Son SM, Lee NK. Changes in upper-extremity muscle activities due to head position in subjects with a forward head posture and rounded shoulders. J Phys Ther Sci 2015;27:1739–1742.ArticlePubMedPMC
- 13. Knox JJ, Hodges PW. Changes in head and neck position affect elbow joint position sense. Exp Brain Res 2005;165:107–113.ArticlePubMedPDF
- 14. Zabihhosseinian M, Yielder P, Holmes MWR, Murphy B. Neck muscle fatigue affects performance of an eye-hand tracking task. J Electromyogr Kinesiol 2019;47:1–9.ArticlePubMed
- 15. León-Hernández JV, Marcos-Lorenzo D, Morales-Tejera D, CuencaMartínez F, La Touche R, Suso-Martí L. Effect of laterality discrimination on joint position sense and cervical range of motion in patients with chronic neck pain: a randomized single-blind clinical trial. Somatosens Mot Res 2019;36:136–143.ArticlePubMed
- 16. Jull GA, O’Leary SP, Falla DL. Clinical assessment of the deep cervical flexor muscles: the craniocervical flexion test. J Manipulative Physiol Ther 2008;31:525–533.ArticlePubMed
- 17. Harris KD, Heer DM, Roy TC, Santos DM, Whitman JM, Wainner RS. Reliability of a measurement of neck flexor muscle endurance. Phys Ther 2005;85:1349–1355.ArticlePubMedPDF
- 18. Kahlaee AH, Rezasoltani A, Ghamkhar L. Is the clinical cervical extensor endurance test capable of differentiating the local and global muscles? Spine J 2017;17:913–921.ArticlePubMed
- 19. Buddenberg LA, Davis C. Test-retest reliability of the Purdue Pegboard test. Am J Occup Ther 2000;54:555–558.ArticlePubMedPDF
- 20. Cabrera-Martos I, Ortiz-Rubio A, Torres-Sánchez I, López-López L, Rodríguez-Torres J, Carmen Valenza M. Agreement between face-to-face and tele-assessment of upper limb functioning in patients with Parkinson disease. PM R 2019;11:590–596.ArticlePubMedPDF
- 21. Klein LJ. Evaluation of the hand and upper extremity. In: Cooper C, editor. Fundamentals of Hand Therapy. 2nd ed. St. Louis: Mosby; 2014:67–86.
- 22. Rusz J, Tykalová T, Krupička R, Zárubová K, Novotný M, Jech R, et al. Comparative analysis of speech impairment and upper limb motor dysfunction in Parkinson’s disease. J Neural Transm (Vienna) 2017;124:463–470.ArticlePubMedPDF
- 23. Alahmari KA, Reddy RS, Silvian PS, Ahmad I, Kakaraparthi VN, Alam MM. Association of age on cervical joint position error. J Adv Res 2017;8:201–207.ArticlePubMedPMC
- 24. Ha SY, Sung YH. A temporary forward head posture decreases function of cervical proprioception. J Exerc Rehabil 2020;16:168–174.ArticlePubMedPMCPDF
- 25. Lee MY, Lee HY, Yong MS. Characteristics of cervical position sense in subjects with forward head posture. J Phys Ther Sci 2014;26:1741–1743.ArticlePubMedPMC
- 26. Alghadir A, Zafar H, Iqbal Z, Al-Eisa E. Effect of sitting postures and shoulder position on the cervicocephalic kinesthesia in healthy young males. Somatosens Mot Res 2016;33:93–98.ArticlePubMed
- 27. Abdelkader NA, Mahmoud AY, Fayaz NA, Saad El-Din Mahmoud L. Decreased neck proprioception and postural stability after induced cervical flexor muscles fatigue. J Musculoskelet Neuronal Interact 2020;20:421–428.PubMedPMC
- 28. Forsyth AL, Joshi RY, Canning CG, Allen NE, Paul SS. Flexed posture in Parkinson disease: associations with nonmotor impairments and activity limitations. Phys Ther 2019;99:893–903.ArticlePubMedPDF
- 29. Alwardat M, Schirinzi T, Di Lazzaro G, Franco D, Imbriani P, Sinibaldi Salimei P, et al. The influence of postural deformities on neck function and pain in patients with Parkinson’s disease. NeuroRehabilitation 2019;44:79–84.ArticlePubMed
- 30. Reddy RS, Meziat-Filho N, Ferreira AS, Tedla JS, Kandakurti PK, Kakaraparthi VN. Comparison of neck extensor muscle endurance and cervical proprioception between asymptomatic individuals and patients with chronic neck pain. J Bodyw Mov Ther 2021;26:180–186.ArticlePubMed
- 31. Wong-Yu ISK, Ren L, Mak MKY. Impaired hand function and its association with self-perceived hand functional ability and quality of life in Parkinson disease. Am J Phys Med Rehabil 2022;101:843–849.ArticlePubMed
- 32. Alonso CCG, de Freitas PB, Pires RS, De Oliveira DL, Freitas SMSF. Exploring the ability of strength and dexterity tests to detect hand function impairment in individuals with Parkinson’s disease. Physiother Theory Pract 2023;39:395–404.ArticlePubMed
- 33. Dahdal P, Meyer A, Chaturvedi M, Nowak K, Roesch AD, Fuhr P, et al. Fine motor function skills in patients with Parkinson disease with and without mild cognitive impairment. Dement Geriatr Cogn Disord 2016;42:127–134.ArticlePubMedPDF
- 34. Roberts HC, Syddall HE, Butchart JW, Stack EL, Cooper C, Sayer AA. The association of grip strength with severity and duration of Parkinson’s: a cross-sectional study. Neurorehabil Neural Repair 2015;29:889–896.ArticlePubMedPDF
- 35. Gillen G, Boiangiu C, Neuman M, Reinstein R, Schaap Y. Trunk posture affects upper extremity function of adults. Percept Mot Skills 2007;104:371–380.ArticlePubMedPDF
- 36. Alwardat M, Di Lazzaro G, Schirinzi T, Sinibaldi Salime P, Mercuri NB, Pisani A. Does Pisa syndrome affect upper limb function in patients with Parkinson’s disease? An observational cross-sectional study. NeuroRehabilitation 2018;42:143–148.ArticlePubMed
- 37. Tabbert H, Ambalavanar U, Murphy B. Neck muscle vibration alters upper limb proprioception as demonstrated by changes in accuracy and precision during an elbow repositioning task. Brain Sci 2022;12:1532.ArticlePubMedPMC
- 38. Treleaven J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man Ther 2008;13:2–11.ArticlePubMed
- 39. Pennington C, Duncan G, Ritchie C. Altered awareness of cognitive and neuropsychiatric symptoms in Parkinson’s disease and dementia with Lewy bodies: a systematic review. Int J Geriatr Psychiatry 2021;36:15–30.ArticlePubMedPDF
- 40. Elangovan N, Tuite PJ, Konczak J. Somatosensory training improves proprioception and untrained motor function in Parkinson’s disease. Front Neurol 2018;9:1053.ArticlePubMedPMC
- 41. Lee MJ, Son JS, Lee JH, Kim SJ, Lyoo CH, Lee MS. Impact of prolonged temporal discrimination threshold on finger movements of Parkinson’s disease. PLoS One 2016;11:e0167034. ArticlePubMedPMC
- 42. Abbruzzese G, Trompetto C, Mori L, Pelosin E. Proprioceptive rehabilitation of upper limb dysfunction in movement disorders: a clinical perspective. Front Hum Neurosci 2014;8:961.ArticlePubMedPMC
- 43. Radosher A, Kalichman L, Moshe S, Ezra D, Simonovich A, Droujin J, et al. Upper quadrant pain and disability associated with a cross-sectional area of deep and superficial neck muscles: a computed tomography study. Spine (Phila Pa 1976) 2022;47:E249–E257.PubMed
- 44. Reece A, Marini F, Mugnosso M, Frost G, Sullivan P, Zabihhosseinian M, et al. Influence of neck pain, cervical extensor muscle fatigue, and manual therapy on wrist proprioception. J Manipulative Physiol Ther 2022;45:216–226.ArticlePubMed
- 45. Seidler RD, Alberts JL, Stelmach GE. Multijoint movement control in Parkinson’s disease. Exp Brain Res 2001;140:335–344.ArticlePubMedPDF
- 46. Farley BG, Sherman S, Koshland GF. Shoulder muscle activity in Parkinson’s disease during multijoint arm movements across a range of speeds. Exp Brain Res 2004;154:160–175.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite