Dear Editor,
Neurodegeneration with brain iron accumulation (NBIA) is a heterogeneous group of inherited neurological disorders characterized by iron deposition in the basal ganglia. Among these disorders, pantothenate kinase-associated neurodegeneration (PKAN) is the most common autosomal-recessive disorder, and it is usually caused by mutations in the pantothenate kinase 2 (PANK2) gene on chromosome band 20p13 [1,2]. PKAN is mainly divided into two phenotypes, classic and atypical [3]. As the phenotypic spectrum of PKAN varies widely, the diagnosis of PKAN can be challenging [1-3]. We report an atypical PKAN case in a patient with a longstanding, unrecognized history of stuttering speech with a misdiagnosis of Parkinson’s disease (PD).
A 54-year-old man diagnosed with PD by his previous neurologist presented to our clinic to establish care. He reported bilateral hand tremor, forceful eye closure, stuttering, slowing, and frequent freezing of gait for the past ten years. Further questioning revealed that he had experienced stuttering speech since his teenage years, although it had worsened in recent years. He had been treated with carbidopa/levodopa for several years with no clear improvement. Upon the review of the patient’s limited medical records from his previous neurologist, results from magnetic resonance imaging (MRI) of the brain performed 7 and 10 years ago indicated no basal ganglia signal abnormality, although MRI imaging was not available to review at the time of the initial evaluation. No family history of similar symptoms was reported. Neurological examination revealed hypomimia, severe blepharospasm, oromandibular dyskinesia, severe stuttering speech/palilalia with frequent speech arrest, bilateral mild postural hand tremor, mild symmetric rigidity, and bradykinesia in all extremities. Gait was bradykinetic with mild stooped posture, bilateral decreased arm swing, and freezing while turning. Laboratory workup included normal complete blood count without acanthocytosis and normal vitamin E, vitamin B12, serum copper, and ceruloplasmin levels.
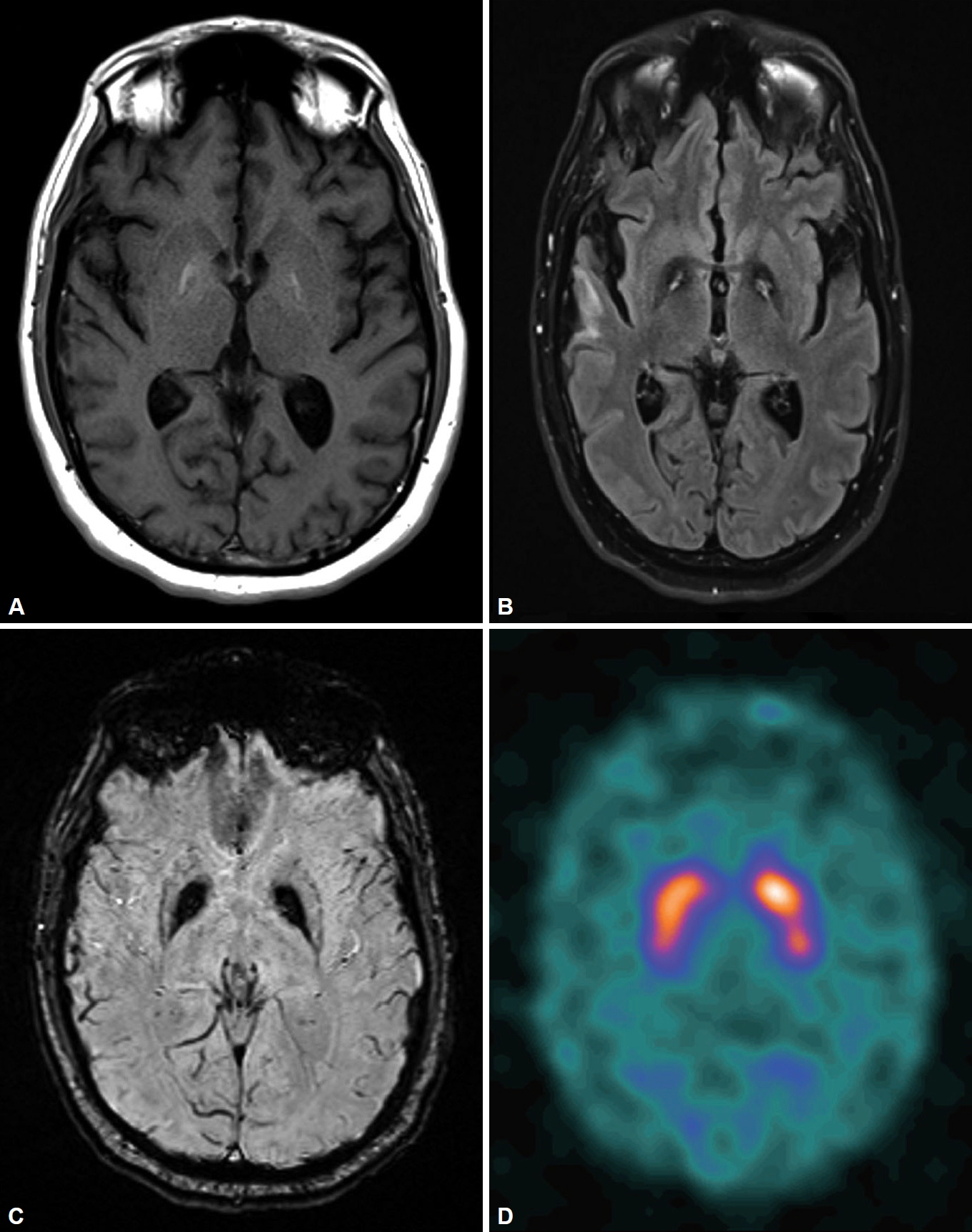
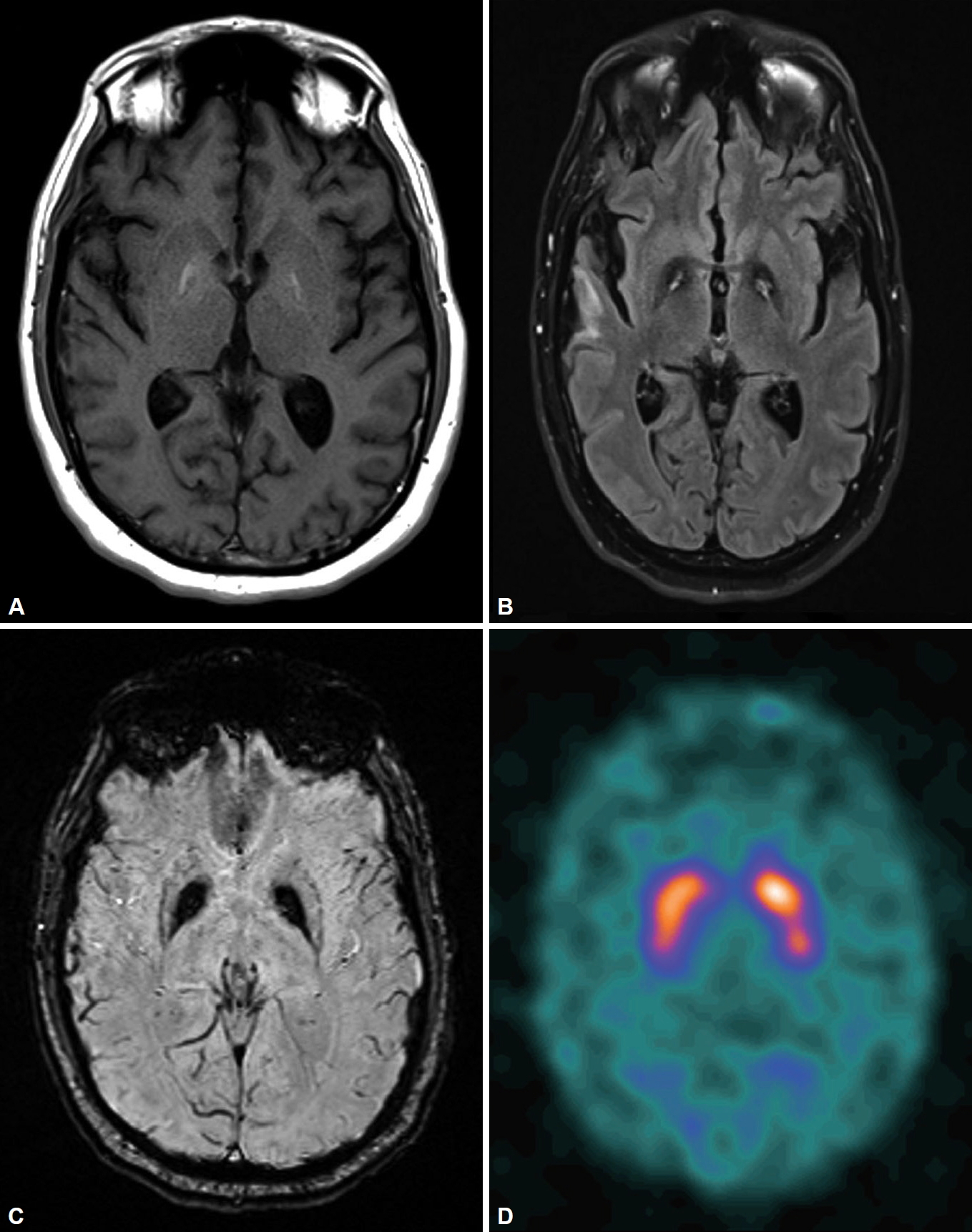
[123I]-Ioflupane-SPECT (DaTscan) results were normal. A repeat brain MRI revealed bilateral symmetric globus pallidus signal abnormalities, including T1 hyperintensity, central foci of hyperintensity with surrounding hypointensity (“eye-of-the-tiger” sign) on fluid attenuated inversion recovery/T2 and dark signals on susceptibility-weighted imaging (Figure 1).
Based on the MRI findings, PANK2 genetic testing was performed, and the results revealed a heterozygous pathogenic variant in exon 6–c.1561G > A (p.Gly521Arg) and a variant of uncertain significance in exon 2–c.795C > A (p.Asp265Glu), strongly supporting a diagnosis of PKAN. Given the lack of a clear response to carbidopa/levodopa as well as normal DaTscan results, his carbidopa/levodopa dose was tapered off. He was started on trihexyphenidyl and amantadine. His blepharospasm was treated with Botox injections with significant improvement.
Retrospectively, his two sets of brain MRI images from an outside imaging center performed 7 years and 10 years prior to his diagnosis of PKAN were retrieved. Although no basal ganglia abnormalities were described in either of the radiology reports, the typical “eye-of-the-tiger” sign was present in both MRIs (Supplementary Figure 1 in the online-only Data Supplement).
PKAN is phenotypically characterized by classic and atypical forms, though there are cases that fall somewhere between. The classic form of the disease has a childhood onset with predominantly extrapyramidal signs (dystonia, rigidity, spasms, Parkinson-like symptoms) [1-3].
Approximately 25% of PKAN patients have a late-onset atypical presentation with speech difficulties (stuttering, palilalia, dysarthria), delayed onset of extrapyramidal motor symptoms (bradykinesia, rigidity, action-induced dystonia, freezing gait, and postural imbalance), seizures and neuropsychiatric symptoms. In a large cohort study of 123 PKAN patients, difficulty with speech, including palilalia (the repetition of words or phrases) and dysarthria, was reported as an unexpected finding in 39% of the patients with atypical PKAN as either the sole presenting feature or part of the early disease [3]. Our patient’s age of onset and the disease course placed him in the atypical PKAN category. However, his heterogenic presentation of early-onset stuttering speech before extrapyramidal motor symptoms had largely been ignored by the patient and his family and was not recognized as a part of the disease process by his previous neurologist during previous evaluations, which contributed to the misdiagnosis of PD.
In PKAN, MR images of the brain are characterized by a distinctive “eye-of-the-tiger” sign, which is diagnostic in most cases. T2-weighted sequences through the basal ganglia show the hypointensity of the globus pallidus with a central hyperintensity, corresponding to excessive brain iron accumulation with gliosis. However, the “eye-of-the-tiger” sign is neither sensitive nor specific, as PKAN cases without the “eye-of-the-tiger” sign have been reported, especially on the initial MRI, as it has also been described in other diseases (mitochondrial membrane-protein associated neurodegeneration, multiple system atrophy, carbon monoxide poisoning, aceruloplasminemia, and neuroferritinopathy) [4-6]. Our patient’s brain MRI results 7 years and 10 years prior to his diagnosis of PKAN were reported by radiologists as showing no basal ganglia abnormalities. However, when images were reviewed retrospectively, both MRIs showed the typical “eye-of-the-tiger” sign, which suggests that radiologist expertise plays an important role in timely recognition of characteristic radiological findings of PKAN.
The DaTscan has been widely used to study presynaptic dopaminergic function and to differentiate presynaptic dopaminergic deficit syndrome (such as PD) from postsynaptic parkinsonian syndromes [7]. However, it has rarely been used for the clinical evaluation of PKAN. A DaTscan was performed as part of our patient’s initial evaluation for an atypical parkinsonian syndrome, and the results indicated grossly normal presynaptic dopamine transporter function, which suggests that PKAN is a postsynaptic parkinsonian syndrome. Retrospectively, our patient would not meet diagnostic criteria for PD even without DaTscan results given his lack of response to levodopa, early bulbar symptoms, and a lack of resting tremor [7]. PANK2 genetic analysis is the gold standard to confirm the diagnosis of PKAN [3].
In summary, although our patient’s history and the MRI “eye-of-the-tiger” sign fit into the diagnosis of PKAN very well, the longstanding unrecognized initial presentation of stuttering speech and the initially missed MRI “eye-of-the-tiger” sign likely contributed to the significant delay in diagnosis, making his case challenging. Clinicians and radiologists should remain vigilant and keep rare hereditary/degenerative parkinsonian syndromes such as NBIA/PKAN in the differential diagnosis when encountering patients presenting with parkinsonism with other hyperkinetic movement disorder features.
Supplementary Material
The online-only Data Supplement is available with this article at https://doi.org/10.14802/jmd.20062.
Supplementary Figure 1.
Brain MR images at 10 years (A) and 7 years (B) prior to the diagnosis of pantothenate kinase-associated neurodegeneration showing bilateral symmetric globus pallidus signal abnormalities consistent with the “eye-of-the-tiger” sign.
jmd-20062-suppl.pdf
Notes
-
Conflicts of Interest
The authors have no financial conflicts of interest.
-
Ethical Standard
Informed consent was obtained from the patient described in the case report.
-
Author Contributions
Conceptualization: Prashant A Natteru. Data curation: Juebin Huang. Formal analysis: Juebin Huang. Methodology: Prashant A Natteru. Supervision: Juebin Huang. Visualization: Juebin Huang. Writing—original draft: Prashant A Natteru. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Acknowledgments
- None.
Figure 1. Brain MRI showing bilateral symmetric globus pallidus signal abnormalities, including T1-weighted imaging hyperintensity (A), the “eye-of-the-tiger” sign on fluid-attenuated inversion recovery imaging (B), and a susceptibility artifact on susceptibility-weighted imaging (C). DaTscan indicating normal uptake (D).
REFERENCES
- 1. Kurian MA, Hayflick SJ. Pantothenate kinase-associated neurodegeneration (PKAN) and PLA2G6-associated neurodegeneration (PLAN): review of two major neurodegeneration with brain iron accumulation (NBIA) phenotypes. Int Rev Neurobiol 2013;110:49–71.ArticlePubMedPMC
- 2. Hogarth P. Neurodegeneration with brain iron accumulation: diagnosis and management. J Mov Disord 2015;8:1–13.ArticlePubMedPMC
- 3. Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, et al. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med 2003;348:33–40.ArticlePubMed
- 4. Diaz N. Late onset atypical pantothenate-kinase-associated neurodegeneration. Case Rep Neurol Med 2013;2013:860201.ArticlePubMedPMCPDF
- 5. Chiapparini L, Savoiardo M, D’Arrigo S, Reale C, Zorzi G, Zibordi F, et al. The “eye-of-the-tiger” sign may be absent in the early stages of classic pantothenate kinase associated neurodegeneration. Neuropediatrics 2011;42:159–162.ArticlePubMed
- 6. Chang CL, Lin CM. Eye-of-the-tiger sign is not pathognomonic of pantothenate kinase-associated neurodegeneration in adult cases. Brain Behav 2011;1:55–56.ArticlePubMedPMC
- 7. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–1601.ArticlePubMed
Citations
Citations to this article as recorded by

- Perspective on investigation of neurodegenerative diseases with neurorobotics approaches
Silvia Tolu, Beck Strohmer, Omar Zahra
Neuromorphic Computing and Engineering .2023; 3(1): 013001. CrossRef - Pantothenate Kinase-Associated Neurodegeneration (PKAN) With Concomitant Blepharospasm: Unveiling a Clinical Enigma
Venkat Reddy, Keyur Saboo, Kavyanjali Reddy, Sunil Kumar, Sourya Acharya
Cureus.2023;[Epub] CrossRef - Enlarged Area of Mesencephalic Iron Deposits in Adults Who Stutter
Jan Liman, Alexander Wolff von Gudenberg, Mathias Baehr, Walter Paulus, Nicole E. Neef, Martin Sommer
Frontiers in Human Neuroscience.2021;[Epub] CrossRef
 , Juebin Huang
, Juebin Huang


 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite