Articles
- Page Path
- HOME > J Mov Disord > Volume 14(3); 2021 > Article
-
Review Article
Emerging Nondopaminergic Medications for Parkinson’s Disease: Focusing on A2A Receptor Antagonists and GLP1 Receptor Agonists -
Pei Shang1
 , Matthew Baker1
, Matthew Baker1 , Samantha Banks2
, Samantha Banks2 , Sa-Ik Hong1
, Sa-Ik Hong1 , Doo-Sup Choi1,3,4
, Doo-Sup Choi1,3,4
-
Journal of Movement Disorders 2021;14(3):193-203.
DOI: https://doi.org/10.14802/jmd.21035
Published online: August 18, 2021
1Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, College of Medicine, Rochester, MN, USA
2Department of Neurology, Mayo Clinic, College of Medicine, Rochester, MN, USA
3Department of Psychiatry and Psychology, Mayo Clinic, College of Medicine, Rochester, MN, USA
4Department of Neuroscience Program, Mayo Clinic, College of Medicine, Rochester, MN, USA
- Corresponding author: Doo-Sup Choi, PhD Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, College of Medicine, Rochester, 200 1st SW, MN 55905, USA / Tel: +1-507-284-5602 / Fax: +1-507-266-0824 / E-mail: choids@mayo.edu
Copyright © 2021 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Parkinson’s disease (PD) is a severe neurodegenerative disease characterized by classic motor features associated with the loss of dopaminergic neurons and appearance of Lewy bodies in the substantia nigra. Due to the complexity of PD, a definitive diagnosis in the early stages and effective management of symptoms in later stages are difficult to achieve in clinical practice. Previous research has shown that colocalization of A2A receptors (A2AR) and dopamine D2 receptors (D2R) may induce an antagonistic interaction between adenosine and dopamine. Clinical trials have found that the A2AR antagonist istradefylline decreases dyskinesia in PD and could be used as an adjuvant to levodopa treatment. Meanwhile, the incretin hormone glucagon-like peptide 1 (GLP1) mainly facilitates glucose homeostasis and insulin signaling. Preclinical experiments and clinical trials of GLP1 receptor (GLP1R) agonists show that they may be effective in alleviating neuroinflammation and sustaining cellular functions in the central nervous system of patients with PD. In this review, we summarize up-to-date findings on the usefulness of A2AR antagonists and GLP1R agonists in PD management. We explain the molecular mechanisms of these medications and their interactions with other neurotransmitter receptors. Furthermore, we discuss the efficacy and limitations of A2AR antagonists and GLP1R agonists in clinical practice.
- Parkinson’s disease (PD) is a progressive and degenerative disorder characterized pathologically by a substantial loss of dopaminergic neurons in the substantia nigra. PD is a relatively wellcharacterized disorder, and objective clinical assessment provides accurate diagnosis and analysis of disease severity. To reverse the dopamine deficiency that drives symptoms in PD, levodopa (L-DOPA), a precursor of dopamine that readily crosses the blood-brain barrier (BBB), revolutionized PD treatment since its Food and Drug Administration (FDA) approval in 1962. Many additional therapeutic drugs used as combinatory treatments work through different mechanisms, such as inhibiting L-DOPA or dopamine metabolism or activating dopamine receptors [1]. Although the efficacy of dopamine-based treatment for PD symptoms is broadly well accepted and tolerable, the fluctuation in dopamine levels in the brain may result in potential side effects, including levodopa-induced dyskinesia (LID), impulsivity, and sleep disturbance [2,3]. Numerous novel drugs have been tested in clinical trials to mitigate dopamine-based treatment shortcomings or reverse or compensate for dopamine deficiency. In 2019, the FDA approved a novel nondopaminergic medication, istradefylline, an adenosine A2A receptor (A2AR) antagonist, as an adjuvant drug to treat off-episodes of PD symptoms [4]. Interestingly, the glucagon-like peptide-1 receptor (GLP1R) agonist exenatide is another emerging nondopaminergic receptor-based treatment option and shows promising preclinical and clinical outcomes. In this review, we discuss how these two G-protein coupled receptor (GPCR) ligands may improve L-DOPA-based treatment with minimized side effects or provide alternative nondopaminergic options for patients who would benefit from early treatment.
INTRODUCTION
- Adenosine and adenosine receptors in movement
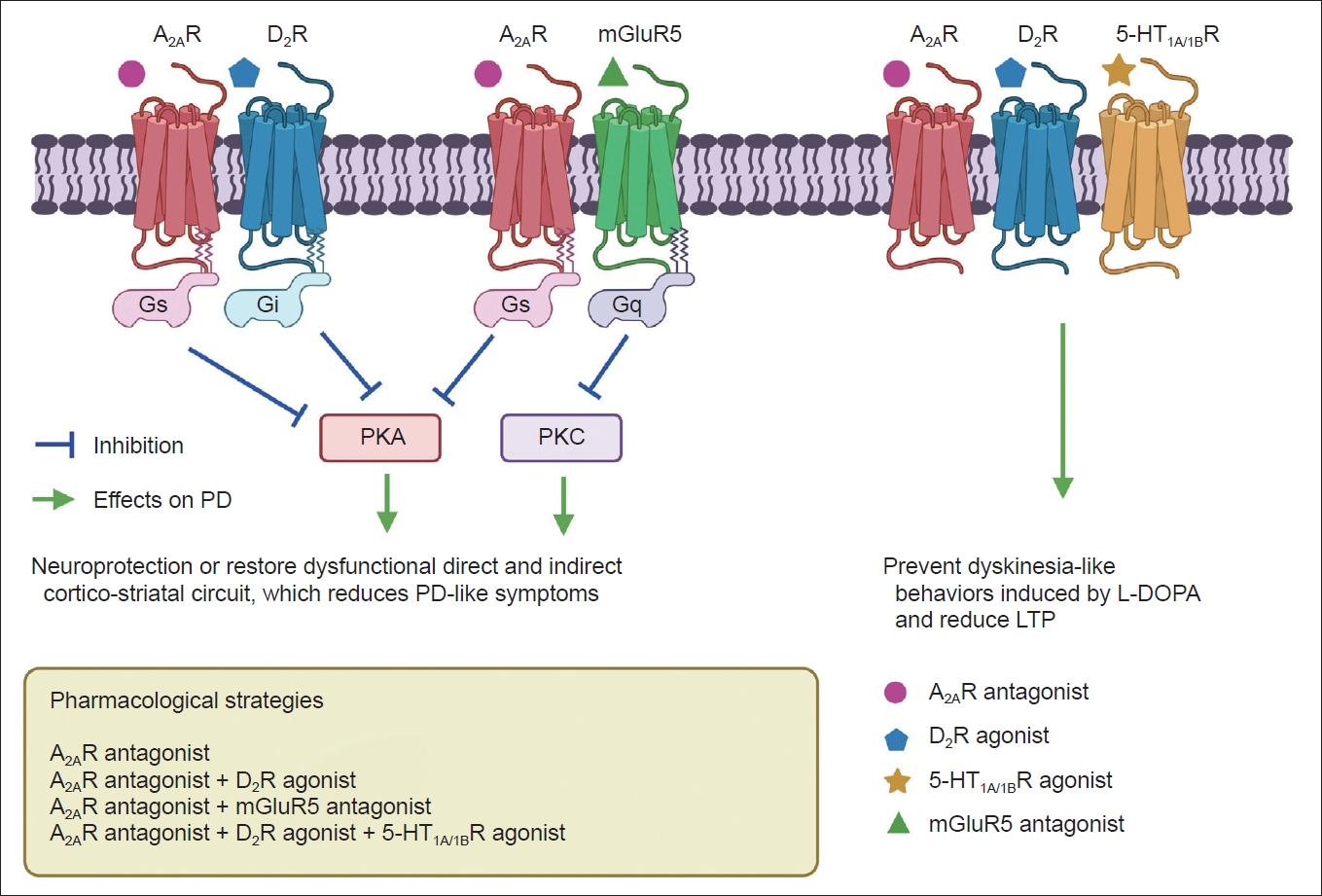
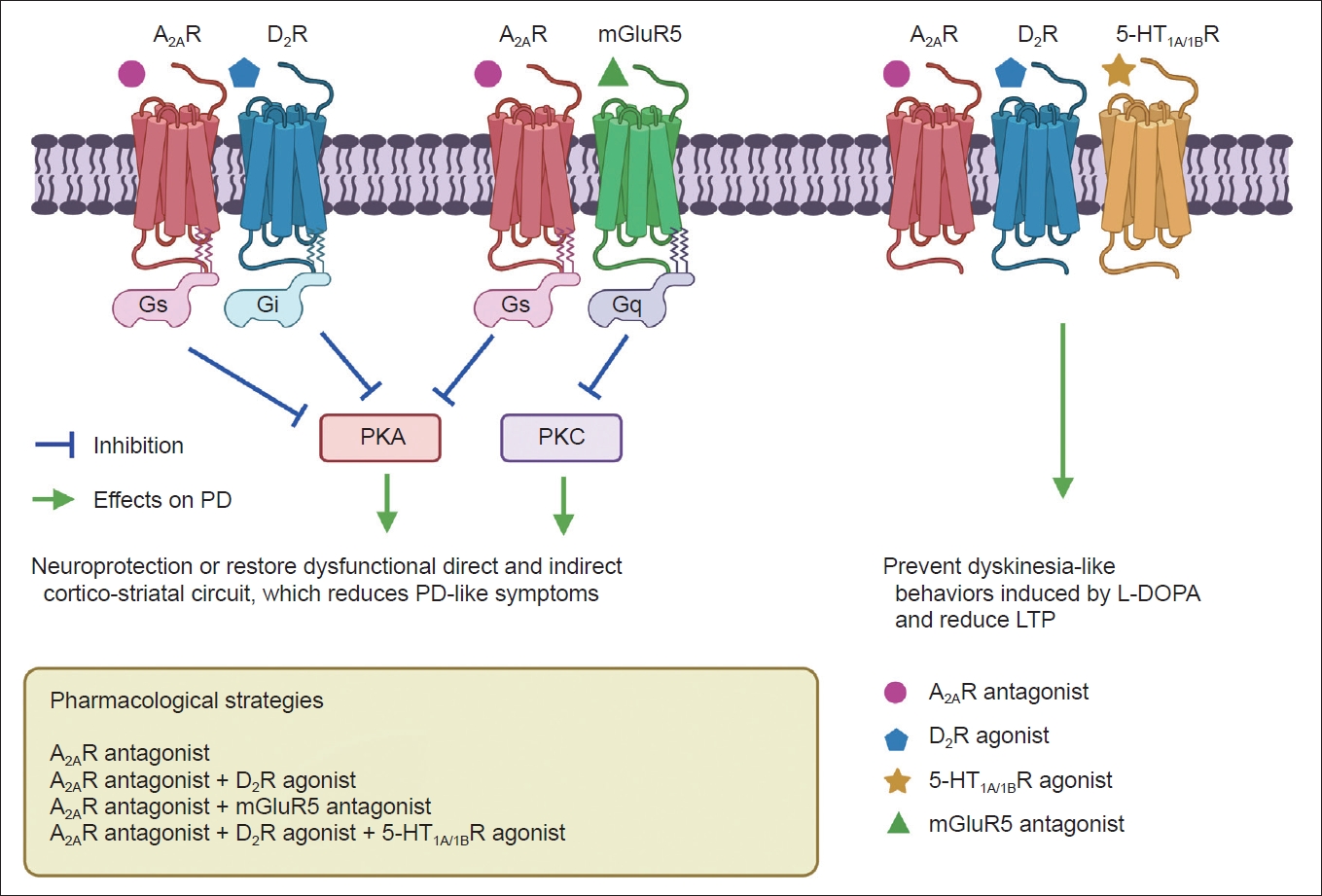
- Adenosine and its receptors have been considered important therapeutic targets for PD due to their neuromodulatory and homeostatic functions in the human brain [5]. Generally, adenosine receptors have been divided into subtypes, including A1, A2A, A2B, and A3. As an endogenous purine nucleoside, adenosine modulates several physiological functions in the central and peripheral nervous systems. Under basal conditions, adenosine mainly acts on inhibitory A1 receptors (A1R) and excitatory A2ARs to integrate dopamine and glutamate signaling, which controls the synaptic plasticity associated with learning, memory, and cognition [5]. Adenosine preferentially acts at A1R because of its widespread distribution and high expression levels [6]. The A2AR is a GPCR that can activate adenylyl cyclase [7]. Of note, A2AR is always distributed and colocalized with dopamine D2R and D3R on striatopallidal neurons in the striatum. A2AR can also interact with dopamine D2R and reduce the expression of D2R (Figure 1) [8,9]. Due to their localization in the basal ganglia, A2AR modulates the indirect pathway by modulating gamma-aminobutyric acid (GABA) and glutamate release, both of which are highly involved in the control of voluntary movements [10].
- Adenosine A2AR antagonists and PD
- D2/D3Rs have long been considered efficient therapeutic agents for the management of PD [11]. However, L-DOPA and its analogs usually function in an early phase of PD, and patients may develop tolerance with long-term treatment [12]. Side effects, including dyskinesias, “on-off” syndromes, and psychotic disorders, can emerge after long-term utilization of dopaminergic agonists [12]. Although surgical interventions such as deep brain stimulation (DBS) can effectively relieve bradykinesia, rigidity, and tremor in PD patients, DBS has several limitations, including the risk of surgical complications and affordability [13]. Istradefylline, an A2AR antagonist, was approved by the FDA as an anti-PD drug in 2019 [14]. Importantly, istradefylline exhibited powerful increases in locomotor activity and potentiated dopaminergic agonist motor effects in animal models of PD (Table 1) [15]. Indeed, dysfunction in adenosinergic transduction is associated with neurological disorders ranging from epilepsy to neurodegenerative disorders [16]. Not surprisingly, the adenosinergic system was identified to be dysregulated in patients with PD [17]. Researchers also demonstrated increased A2AR density in the caudate-putamen from PD subjects in a postmortem study [18,19]. Consistently, pharmacological inhibition by A2AR antagonists has been found to improve motor behavior deficits in PD and dyskinesia [20]. Interestingly, a photoactive adenosine A2AR antagonist also showed potential to remotely control movement disorders such as PD [21].
- A2AR antagonism was first identified for potential therapeutic effects in patients with PD with the antimalarial drug mefloquine, which contains an A2AR antagonist [22]. Experimentally, periodically interrupted or long-lasting administration of an A2AR antagonist such as SCH58261 combined with L-DOPA may restore normal motor function in PD animal models [23]. Interestingly, long-acting L-DOPA treatment also results in an enhanced biosynthesis of A2ARs in patients with PD-induced dyskinesias, especially in brain networks involving the striatum and substantia nigra [24].
- Interactions between adenosine A2AR and other GPCRs in PD
- The hypothesis for A2AR antagonist utilization in PD is based on adenosine-dopamine antagonism in the striatum, which has the highest expression of A2AR in the human body [25,26]. Accumulated evidence has verified the functional relationships between A2AR and D2R in the basal ganglia (Figure 1) [27,28]. Researchers have also identified D2R/A2AR oligomers in the mouse and monkey striatum via proximity ligation assays, immunoelectron microscopy, and ligand fluorescence resonance energy transfer (FRET)-based approaches [29-31]. In addition, D2R/A2AR oligomers in the striatum have been postulated to appear at the onset of PD and could interrupt selective dopaminergic denervation [32]. Furthermore, A2AR mRNA has been detected in striatal cholinergic interneurons according to a previous study [33]. Due to structural associations, A2AR activation can inhibit Gi/o protein (G protein α subunit, which inhibits adenylyl cyclase activity and thereby decreases cAMP levels) coupled with D2R, further validating the existence of the A2AR-D2R heteroreceptor complex [34,35].
- Meanwhile, D2R activation can lead to the formation of D2R-NMDAR heteroreceptor complexes and subsequent inhibition of NMDAR signaling [36]. Overactivity of astroglial A2AR induces extrasynaptic and postsynaptic mGluR1 and mGluR5 coupling to Gq protein (G protein α subunit, which activates phospholipase β and thereby increases diacylglycerol and inositol-triphosphate levels) by enhancing astroglial glutamate release, which can further increase intracellular calcium levels and lead to inhibition of D2R signaling in A2AR-D2R and A2AR-mGluR5 complexes (Figure 1) [37]. The A2AR-D2R and A2AR-mGluR5 complexes may inhibit D2R promoter recognition and activate intracellular MAPK and CREB signaling pathways, mainly relying on antagonistic allosteric receptor-to-receptor interactions [38,39]. Nonetheless, A2AR activation by adenosine may have an excitatory effect on striatopallidal neurons in a D2R-independent manner [40,41].
- During the early phase of PD, L-DOPA and D2R agonists can prevent D2R promoter inhibition induced by the basal A2AR promoter since more D2R homoreceptor complexes are expressed than A2AR-D2R complexes [42]. 5-HT1A/1B agonists combined with A2AR antagonists were also reported as an advanced therapy for PD with antidyskinetic effects, implicating a potential synergic effect between the two receptors (Figure 1) [43].
- Adenosine A2AR antagonists on cognition
- Cognitive impairment frequently occurs in patients with PD and usually reduces patient quality of life and comfort [44]. Early cognitive deficits in PD were hypothesized to be associated with deficits in dopaminergic innervation of the cortex and alterations in striatum-thalamocortical loop function, which is difficult to manage using dopaminergic medications [45,46]. Interestingly, A2AR antagonists improve cognitive functions, including memory. The A2AR antagonist SCH58261 improved memory performance and social recognition memory in rodent models with memory deficits [47]. However, A2AR antagonist administration into the posterior cingulate cortex impaired the process of memory retrieval in rats [48], suggesting region-specific effects. In practice, donepezil, an acetylcholinesterase inhibitor, is commonly prescribed for patients with PD to manage cognitive impairment and memory loss and might be an adjuvant to A2AR antagonists to alleviate side effects [49].
- A2AR antagonists, including ZM241385 and istradefylline, may restore social recognition and cognitive deficits in rats [50,51]. Similarly, A2AR antagonists or genetic deletion of A2AR were shown to improve short-term memory, working memory, reversal learning, goal-directed behavior, and fear conditioning in animal models used for different neurological diseases [51-56].
- Adenosine A2AR antagonists: efficacy and limitations
- It has been clinically verified that A2AR antagonists can improve motor dysfunction in patients with PD as monotherapy or in combination with L-DOPA and other antiparkinsonian drugs (Table 1) [57,58]. Previous clinical trials have also shown that A2AR antagonists are effective in shortening the off-time without worsening troublesome dyskinesia and increasing on-time in patients with advanced stage PD and L-DOPA treatment [59]. However, with the exception of istradefylline, almost all of the other clinical trials with novel therapeutics have failed in recent years, including preladenant, vipadenant, and the nonxanthine SCH58261 [59,60]. Clinical trials of the A2AR antagonist named preladenant were discontinued due to the lack of efficacy [61]. Side effects induced by A2AR antagonists, including insomnia, headache, constipation, hallucinations, and cardiac failure, merit attention from caregivers [62]. Targeting A2AR with classic pharmacology has shown some drawbacks, including slow and imprecise drug delivery and low specificity and efficacy [21]. A2AR antagonists generally have higher molecular weights and are difficult to synthesize due to the complexity of structures, poor water solubility, and furan groups that preclude replacement by classic chemistry [63]. Caffeine is an adenosine analog and has been shown to confer neuroprotection against dopaminergic neurodegeneration via modulation of A2AR pathways and neuroinflammation in PD models [64]. Furthermore, caffeine was demonstrated to improve motor function in PD patients by targeting A2AR [65]. Chronic caffeine treatment can largely attenuate α-synuclein-induced microglial activation and astrogliosis in mice, similar to A2AR antagonists [66,67]. However, a clinical trial has shown that caffeine intake twice daily (200 mg) over 6 months did not produce significant symptomatic benefits for patients with PD [68]. Therefore, further studies are required to clarify the benefits of caffeine for the prevention or improvement of early or moderate PD symptoms.
- Interestingly, our preclinical experiment showed that A2AR inhibition increases alcohol-seeking behaviors [69] through enhanced goal-directed cognitive function. Consistent with this finding, pharmacological activation of A2AR or optogenetic activation of A2AR-expressing neurons in the dorsomedial striatum (DMS) decreases alcohol-seeking behaviors [70]. In corticostriatal circuits, A2AR-expressing neurons consist of indirect and inhibiting circuits, as discussed in the previous section. Adenosine is known to mediate the intoxicating effect of alcohol [71-75], and A2AR inhibition may increase reward-seeking behaviors when subjects are introduced to addictive substances or activities. As noted above, A2AR inhibition increases cognitive function through enhanced goal-directed behavior. Even though istradefylline was suspected to positively affect cognitive dysfunction and postural abnormalities in patients with PD, short-term clinical trials did not show benefits on cognition [76]. Meanwhile, based on preclinical studies, A2AR inhibition may increase the risk of addiction. Therefore, it is important to monitor the behavioral patterns of PD patients when A2AR antagonists are prescribed.
ADENOSINE A2AR ANTAGONISTS
- Glucagon-like peptide and GLP1R-mediated signaling in insulin regulation
- Glucagon-like peptide-1 (7-36) amide (GLP1) is secreted from intestinal enteroendocrine L cells in response to food intake and controls systemic blood glucose homeostasis in the human body [77]. The intestinal wall secretes GLP1 to activate enteroenteric reflexes, control gastric motility, and slow gastric emptying [78]. GLP1 also activates vagal sensory nerve terminals and initiates vagal-vagal autonomic reflexes by controlling the endocrine function of the pancreas [79]. The islets of Langerhans in the pancreas can be stimulated by GLP1 and release insulin to inhibit glucagon production. GLP1Rs are usually expressed in hypothalamic neurons and vagal afferent ganglion neurons [80]. Due to rapid degradation and short half-lives, only 10–15% of GLP1 reaches circulation after release [81]. Peripheral injection of GLP1R antagonists facilitates food intake and diminishes the efficacy of circulating GLP1, suggesting that the feeding process induced by GLP1 relies on peripheral GLP1R, which activates vagal afferents after stimulation [82,83]. Notably, the glucose-lowering function of GLP1 is highly dependent on the concentration of glucose [84]. This property of GLP1 prevents it from lowering blood glucose after fasting [85]. Therefore, GLP1R agonists are clinically used as a new class of glucose-controlling agents for treating type 2 diabetes (T2D) without introducing the side effects of hypoglycemia [86,87].
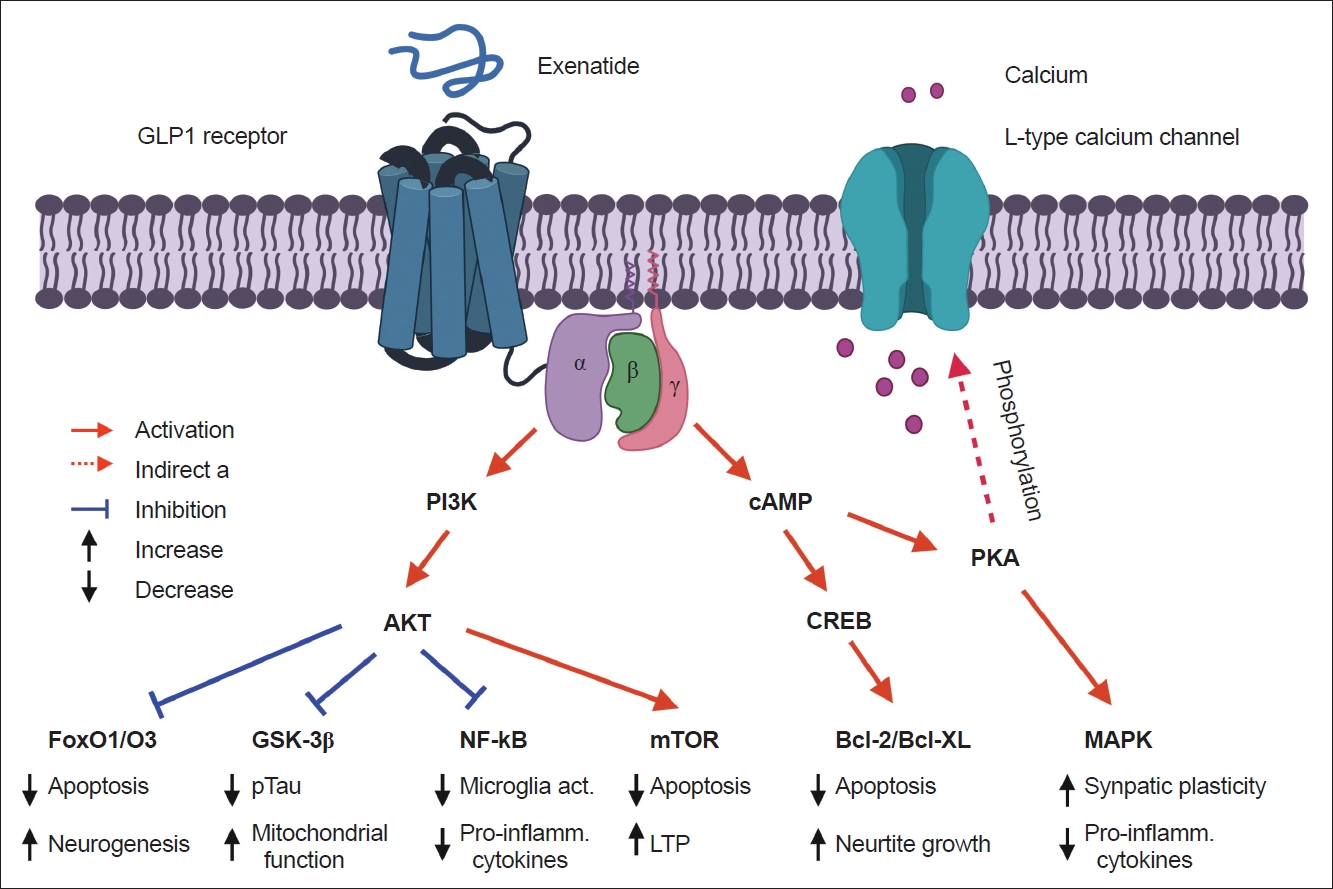
- Based on previous findings, GLP1 may not influence the metabolome or directly interact with rodent β cells, and short-term exposure to GLP1 did not induce changes to glycolytic or TCA cycle intermediates in vitro [88,89]. The binding between GLP1 and GLP1R on various cells can activate adenylyl cyclase and increase cAMP levels, which further stimulates protein kinase A and cAMP-regulated guanine nucleotide exchange factor 2 pathways and insulin secretion [90,91]. A previous study also found that GLP1 may stimulate the secretion of insulin in β cells and induce glucose catabolism via the mTOR-dependent HIF-1α activation pathway (Figure 2) [92].
- As a promising therapeutic strategy for the management of T2D, GLP1 efficacy has been compared with different insulin formulations, including glargine and detemir, in multiple clinical trials [93,94]. Long-acting GLP1R agonists have shown better glycemic efficacy than basal insulin. For instance, a once-weekly regimen of semaglutide significantly decreased the HbA1c levels compared with glargine therapy [95].
- GLP1R and dopaminergic neurons
- While GLP1R plays an important peripheral role in glucose-dependent insulin secretion and gene expression, there is increasing evidence of its central role in feeding and satiety-related behaviors. GLP1R expression has been observed throughout the brain in both rodents and humans. In particular, GLP1R is highly expressed in mesolimbic reward pathways, including the hypothalamus, ventral tegmental area, lateral septum, nucleus of the solitary tract, and many others. GLP1 and other GLP1R agonists have been shown to cross the BBB. GLP1R is a Gα-coupled GPCR that activates adenylyl cyclase, leading to increased intracellular cAMP levels (Figure 2). GLP1R activation has been shown to directly interact with the dopamine system by decreasing phasic dopamine release and facilitating a reduction in feeding and reward-seeking behaviors [96]. Additionally, GLP1R stimulation has been shown to exert neuroprotective and neuroproliferative effects in response to stroke, neurodegeneration, and other neurologic injuries (Figure 2).
- In particular, exenatide, a novel GLP1R agonist that was discovered in the saliva of the Gila monster (Heloderma suspectum), was shown to reduce dopaminergic cell loss in the substantia nigra in a methyl-phenyl tetrahydropyridine (MPTP)-induced mouse model of PD and restore normal dopamine levels and motor function [97]. Similarly, exenatide treatment was shown to reduce dopaminergic cell loss and motor function in rats injected with 6-hydroxydopamine (6-OHDA), another toxin that selectively causes dopaminergic cell death. In addition to motor effects, GLP1R has been shown to have a neuroprotective role in cognitive function through enhanced synaptic plasticity. While GLP1R null mouse models have shown evidence of impaired long-term potentiation (LTP) and learning and memory deficits, GLP1R stimulation increases LTP and restores cognitive function in neurodegenerative mouse models.
- The extended-release form of the protease-resistant and brain-penetrating exenatide was approved by the FDA for T2D in 2018. Following this, Dr. Foltynie’s group in England demonstrated that weekly administration of exenatide for 48 weeks significantly improved PD symptoms in a phase II clinical trial [98]. Recently, improved extended-release forms of exenatide with increased brain penetration and longer bioavailability were developed [99-101]. Since various forms of exenatide were comprehensively tested in humans through T2D clinical studies, the new application of exenatide in PD will be available for PD treatment in the near future.
- GLP1R agonists: efficacy and limitations
- GLP1 function is hypothesized to be a pivotal molecular pathway in glucose regulation via the gut-brain axis. Interestingly, GLP1R is expressed not only in peripheral organs such as the pancreas but also in the brain [97,102-104]. In line with this theory, GLP1R agonists showed positive impacts on controlling cardiovascular diseases, especially in patients with diabetes [105,106]. Liraglutide, a GLP1R agonist, has been implicated as an efficient weight-loss agent in patients with or without T2D [107]. GLP1R agonists such as exenatide have also been demonstrated to exert neuroprotective and neurotrophic effects and have shown therapeutic efficacy in PD management in several clinical and preclinical trials (Table 2 and 3) [98,108]. Another GLP1R agonist, lixisenatide, can cross the BBB and increase cAMP levels at a low dose [109]. GLP1R activation elicited neurite outgrowth of SH-SY5Y cells, similar to the function of nerve growth factors, further validating its potential role in neurogenesis and neurotrophy [110]. Meanwhile, exenatide induces approximately twofold changes in doublecortin-positive cells in the medial striatum and bromodeoxyuridine-positive cells in the subventricular area in adult mice, both of which are highly involved in the process of neurogenesis [111]. Exenatide also improves Mattis dementia rating scale scores in patients with PD [108]. The follow-up study showed that the benefits lasted for 12 months after the cessation of exenatide [112,113].
- The neuroprotective mechanisms of GLP1R agonists have not been elucidated until now. The brain-penetrant long-acting GLP1R agonist NLY01 prevented the loss of dopaminergic neurons and improved behavioral deficits in an α-synuclein-treated fibril model mimicking sporadic PD [114,115]. NLY01 prolonged life expectancy and alleviated neurodegeneration and neuropathological abnormalities in the human A53T α-synuclein (hA53T) transgenic mouse model [116]. The neuroprotective effects of the GLP1R agonist are likely correlated with the MAPK (ERK) and PI3K/AKT pathways (Figure 2) [90,117].
GLP1R AGONISTS
- This review provides current perspectives on the recently approved istradefylline and the recently investigated exenatide as new treatment options for PD. Ongoing postmarketing clinical studies on istradefylline will reveal optimal dosing and treatment timing for PD symptom management. Regarding exenatide, as it is clinically used for T2D, additional trials will inform the potential benefits in PD and T2D. Furthermore, various suspended release forms of exenatide will be available for once weekly or every other week treatment regimens. More user-friendly routes of administration (pencil-type syringe or oral form) will increase the accessibility of this novel medication. Importantly, we need to develop personalized treatment methods for PD based on more precise phenotyping and genotyping, which may correlate with the treatment outcomes associated with existing and novel medications. Big-data-driven artificial intelligence will eventually aid physicians’ treatment strategies with improved symptom monitoring systems.
CONCLUSIONS
-
Conflicts of Interest
D.S.C. is a scientific advisory board member for Peptron Inc. Peptron had no role in the preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication. All the other authors declare no biomedical financial interests or potential conflicts of interest.
-
Funding Statement
This work was supported by the Samuel C. Johnson for Genomics of Addiction Program at Mayo Clinic, the Ulm Foundation, and National Institute on Alcohol Abuse and Alcoholism (K01 AA027773 to SK; R01 AA018779, R01 AA029258, and R01 AG072898 to DSC).
-
Author Contributions
Conceptualization: Pei Shang, Doo-Sup Choi. Funding acquisition: Doo-Sup Choi. Investigation: all authors. Project administration: Pei Shang, Doo-Sup Choi. Resources: Pei Shang, Matthew Baker, Samantha Banks, Doo-Sup Choi. Supervision: Doo-Sup Choi. Writing—original draft: all authors. Writing—review & editing: all authors.
Notes
- We thank all the Choi laboratory members for their discussions. Figure 1 and 2 were created with BioRender.com.
Acknowledgments
| Medication | Trial design | Subjects | Treatment doses | Outcomes | Reference |
|---|---|---|---|---|---|
| Istradefylline | A phase 2, 12-week, double-blind, placebo-controlled study of istradefylline in PD patients on L-DOPA/carbidopa | 790 PD patients with an average OFF time at least 2 h/day and approximately 3.2 years after diagnosis | 20 (for 163 subjects) or 60 mg (for 155 subjects) per day | Significant reduction in the awake time per day spent in OFF state | [119] |
| Istradefylline | A phase 3, multicenter, open-label, long-term (52 w) study of istradefylline in PD patients experiencing wearing-off | 313 PD patients approximately 7.5 years after onset and 3.3 years after showing motor complications | 20 mg as starting dosage with/o adjustment to 40 mg | Significant OFF time reduction since the 2nd week | [120] |
| Istradefylline | Istradefylline as adjunctive treatment to levodopa for 12 weeks in a phase 3, double-blind manner in PD patients with motor complications | 373 PD patients 3.3 years after showing motor complications | 20 or 40 mg per day | Istradefylline markedly reduced daily OFF time and was well-tolerated in patients with motor complications | [121] |
| Istradefylline | A phase 3 randomized, 12-week, double-blind, placebo-controlled parallel-group study of istradefylline with different doses in patients on levodopa therapy | 610 PD patients with an average OFF time at least 3 h/day. 9 years after diagnosis, and 3.6 years after motor fluctuations | 10, 20, and 40 mg per day | Istradefylline did not impact daily OFF time but significantly improved motor scores at 40 mg per day | [122] |
| Tozadenant | A phase 2, double-blind, randomized, placebo-controlled study of the safety and efficacy of SYN115 as adjunctive therapy in L-DOPA- treated PD subjects | 337 PD patients with an average OFF time at least 6 h/day and 8.7 years after diagnosis | 60 or 120 or 180 or 240 mg/BID | Tozadenant significantly reduced daily OFF time and improved motor signs without increasing dyskinesia | [123] |
| Preladenant | A phase 2, 36-week, open-label, follow-up safety study of SCH420814 in subjects with PD | 140 PD patients with moderate to severe PD > 5 years | 5 mg/BID | Long-term preladenant treatment are well-tolerated and sustained the OFF time reduction | [124] |
| GLP1R agonist | PD models | Treatment details | Experimental results | Reference |
|---|---|---|---|---|
| Ex-4 | 6-OHDA/LPS-treated rat model | Ex-4 (0.1 and 0.5 µg/kg) was given 7 days after the intracerebral toxin injection, BID for 7 days | Circling behavior was attenuated in Ex-4 group; striatal tissue dopamine level increased in Ex-4 group | [125] |
| Ex-4 | 6-OHDA-treated rat model | Ex-4 (0.1 μg/kg) was given 5 weeks after the intracerebral toxin injection, BID for 21 days | Ex-4 promoted neurogenesis and normalized the imbalance in dopamine levels; Ex-4 also increased the dopaminergic neurons in substantia nigra | [111] |
| Ex-4 | MPTP-treated mouse model | Ex-4 (20 nM, 0.25 μL/h) was given 7 days 2 hour before MPTP treatment via left ventricle administration | Ex-4 protected dopaminergic neurons, preserved dopamine levels and improved motor functions | [97] |
| Extended-release Ex-4 (PT302) | 6-OHDA-treated rat model | Ex-4 (0.4 or 2 mg/kg) was given every 2 weeks for 10 weeks starting 16 days before the unilateral lesion induced by 6-OHDA | PT302 increased tyrosine hydroxylase levels in the lesioned substantia nigra and striatum; PT302 reduced the neurodegeneration of nigrostriatal dopaminergic neurons | [126] |
| Lixisenatide | MPTP-treated mouse model | Lixisenatide (10 nmol/kg) was given after the 7-day MPTP treatment, once a day for 14 days | Lixisenatide prevented MPTP-induced motor impairment, reduction in tyrosine hydroxylase levels in substantia nigra, and reduction in pro-apoptotic signaling | [127] |
| GLP1R agonist | Treatment details | Subjects | Study design | Primary outcome measures | Conclusions | Reference |
|---|---|---|---|---|---|---|
| Ex-4 | Self-administer twice-daily subcutaneous injections of 5 µg for 1 month and 10 µg for 11 months | 45 patients: moderate PD approximately 7.5 years since disease onset | Single-blind, placebo-controlled. 21-Ex-4 and 24-placebo | MDS-UPDRS and nonmotor tests at baseline, 6 months, 12 months, and 14 months | MDS-UPDRS scores in the Ex-4 treated group improved 2.7 points compared with 2.2 in the control group; motor and cognitive functions also improved in Ex-4 treated group | [108] |
| Ex-4 | Once-weekly subcutaneous injections of 2 mg for 48 weeks | 62 patients: moderate PD with DAergic treatment with wearing-off effects | Single-center, randomized, double-blind, placebo-controlled. 32-Ex-4 and 30-placebo. | MDS-UPDRS motor subscale (part 3) | Ex-4 significantly improved MDS-UPDRS scores of patients in the OFF time | [98] |
| Ex-4 | 2 mg of once weekly or placebo for 48 weeks followed by a 12-week washout period | 60 patients: moderate PD; patients were receiving dopaminergic treatment | Single-center, randomized, double-blind, placebo-controlled. 31-Ex-4 and 29-placebo | MDS-UPDRS and serum were collected after 12-week withdrawal; insulin and PKB-related pathways were tested | Ex-4 treated group showed increased phospho-IRS1 and elevated expression of total AKT and phospho-mTOR; improvement of MDS-UPDRS was correlated to total and phospho-mTOR level | [113] |
Ex-4: exenatide-4, mTOR: mechanistic target of rapamycin, IRS1: insulin receptor substrate 1, MDS-UPDRS: Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale, PD: Parkinson’s disease, PKB: protein kinase B, DAergic: dopaminergic, GLP1R: glucagon-like peptide-1 receptor.
- 1. Charvin D, Medori R, Hauser RA, Rascol O. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat Rev Drug Discov 2018;17:844.ArticlePubMed
- 2. Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 2015;132:96–168.ArticlePubMed
- 3. Moore TJ, Glenmullen J, Mattison DR. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med 2014;174:1930–1933.ArticlePubMed
- 4. Chen JF, Cunha RA. The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal 2020;16:167–174.ArticlePubMedPMC
- 5. Chen JF. Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol 2014;119:257–307.ArticlePubMed
- 6. Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 2001;38:107–125.ArticlePubMed
- 7. Zheng J, Zhang X, Zhen X. Development of adenosine A2A receptor antagonists for the treatment of Parkinson’s disease: a recent update and challenge. ACS Chem Neurosci 2019;10:783–791.ArticlePubMed
- 8. Morelli M, Blandini F, Simola N, Hauser RA. A(2A) receptor antagonism and dyskinesia in Parkinson’s disease. Parkinsons Dis 2012;2012:489853.PubMedPMC
- 9. Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 2003;278:46741–46749.PubMed
- 10. Morelli M, Carta AR, Jenner P. Adenosine A2A receptors and Parkinson’s disease. Handb Exp Pharmacol 2009;589–615.Article
- 11. Jenner P. A2A antagonists as novel non-dopaminergic therapy for motor dysfunction in PD. Neurology 2003;61:S32–S38.ArticlePubMed
- 12. Morelli M, Wardas J. Adenosine A(2a) receptor antagonists: potential therapeutic and neuroprotective effects in Parkinson’s disease. Neurotox Res 2001;3:545–556.ArticlePubMed
- 13. Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 2007;130:1596–1607.ArticlePubMed
- 14. Hussar DA. New drugs 2020, part 1. Nursing 2020;50:31–38.Article
- 15. Bibbiani F, Oh JD, Petzer JP, Castagnoli N Jr, Chen JF, Schwarzschild MA, et al. A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson’s disease. Exp Neurol 2003;184:285–294.ArticlePubMed
- 16. Ribeiro JA, Sebastião AM, de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 2002;68:377–392.ArticlePubMed
- 17. McFarland NR, Burdett T, Desjardins CA, Frosch MP, Schwarzschild MA. Postmortem brain levels of urate and precursors in Parkinson’s disease and related disorders. Neurodegener Dis 2013;12:189–198.ArticlePubMed
- 18. Varani K, Vincenzi F, Tosi A, Gessi S, Casetta I, Granieri G, et al. A2A adenosine receptor overexpression and functionality, as well as TNF-α levels, correlate with motor symptoms in Parkinson’s disease. FASEB J 2010;24:587–598.ArticlePubMed
- 19. Villar-Menéndez I, Porta S, Buira SP, Pereira-Veiga T, Díaz-Sánchez S, Albasanz JL, et al. Increased striatal adenosine A2A receptor levels is an early event in Parkinson’s disease-related pathology and it is potentially regulated by miR-34b. Neurobiol Dis 2014;69:206–214.ArticlePubMed
- 20. Pinna A, Serra M, Morelli M, Simola N. Role of adenosine A(2A) receptors in motor control: relevance to Parkinson’s disease and dyskinesia. J Neural Transm (Vienna) 2018;125:1273–1286.ArticlePubMed
- 21. Taura J, Nolen EG, Cabré G, Hernando J, Squarcialupi L, López-Cano M, et al. Remote control of movement disorders using a photoactive adenosine A(2A) receptor antagonist. J Control Release 2018;283:135–142.ArticlePubMedPMC
- 22. Weiss SM, Benwell K, Cliffe IA, Gillespie RJ, Knight AR, Lerpiniere J, et al. Discovery of nonxanthine adenosine A2A receptor antagonists for the treatment of Parkinson’s disease. Neurology 2003;61:S101–S106.ArticlePubMed
- 23. Carta AR, Pinna A, Tronci E, Morelli M. Adenosine A2A and dopamine receptor interactions in basal ganglia of dopamine denervated rats. Neurology 2003;61:S39–S43.ArticlePubMed
- 24. Calon F, Dridi M, Hornykiewicz O, Bédard PJ, Rajput AH, Di Paolo T. Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain 2004;127(Pt 5):1075–1084.ArticlePubMed
- 25. Fuxe K, Strömberg I, Popoli P, Rimondini-Giorgini R, Torvinen M, Ogren SO, et al. Adenosine receptors and Parkinson’s disease. Relevance of antagonistic adenosine and dopamine receptor interactions in the striatum. Adv Neurol 2001;86:345–353.PubMed
- 26. Josselyn SA, Beninger RJ. Behavioral effects of intrastriatal caffeine mediated by adenosinergic modulation of dopamine. Pharmacol Biochem Behav 1991;39:97–103.ArticlePubMed
- 27. Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev 2003;55:509–550.ArticlePubMed
- 28. Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 1997;20:482–487.ArticlePubMed
- 29. Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, et al. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 2011;51:111–118.ArticlePubMedPMC
- 30. Bonaventura J, Rico AJ, Moreno E, Sierra S, Sánchez M, Luquin N, et al. L-DOPA-treatment in primates disrupts the expression of A(2A) adenosine-CB(1) cannabinoid-D(2) dopamine receptor heteromers in the caudate nucleus. Neuropharmacology 2014;79:90–100.ArticlePubMed
- 31. Fernández-Dueñas V, Taura JJ, Cottet M, Gómez-Soler M, López-Cano M, Ledent C, et al. Untangling dopamine-adenosine receptor-receptor assembly in experimental parkinsonism in rats. Dis Model Mech 2015;8:57–63.PubMed
- 32. Fuxe K, Marcellino D, Borroto-Escuela DO, Guescini M, Fernández-Dueñas V, Tanganelli S, et al. Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 2010;16:e18–e42.ArticlePubMedPMC
- 33. Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol 1996;118:1461–1468.ArticlePubMedPMC
- 34. Borroto-Escuela DO, Marcellino D, Narvaez M, Flajolet M, Heintz N, Agnati L, et al. A serine point mutation in the adenosine A2AR C-terminal tail reduces receptor heteromerization and allosteric modulation of the dopamine D2R. Biochem Biophys Res Commun 2010;394:222–227.ArticlePubMed
- 35. Borroto-Escuela DO, Romero-Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO, et al. G protein-coupled receptor heterodimerization in the brain. Methods Enzymol 2013;521:281–294.ArticlePubMed
- 36. Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron 2006;52:897–909.ArticlePubMed
- 37. Cabello N, Gandía J, Bertarelli DC, Watanabe M, Lluís C, Franco R, et al. Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J Neurochem 2009;109:1497–1507.ArticlePubMedPMC
- 38. Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueño J, Gutiérrez MA, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A 2002;99:11940–11945.ArticlePubMedPMC
- 39. Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, et al. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology 2003;61:S19–S23.ArticlePubMed
- 40. Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol 1999;59:355–396.ArticlePubMed
- 41. Svenningsson P, Lindskog M, Rognoni F, Fredholm BB, Greengard P, Fisone G. Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience 1998;84:223–228.ArticlePubMed
- 42. Fuxe K, Borroto-Escuela DO. Heteroreceptor complexes and their allosteric receptor–receptor interactions as a novel biological principle for integration of communication in the CNS: targets for drug development. Neuropsychopharmacology 2016;41:380–382.ArticlePubMed
- 43. Pinna A, Ko WK, Costa G, Tronci E, Fidalgo C, Simola N, et al. Antidyskinetic effect of A2A and 5HT1A/1B receptor ligands in two animal models of Parkinson’s disease. Mov Disord 2016;31:501–511.ArticlePubMed
- 44. Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol 2012;11:697–707.ArticlePubMed
- 45. Narayanan NS, Rodnitzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci 2013;24:267–278.ArticlePubMed
- 46. Robbins TW, Cools R. Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Mov Disord 2014;29:597–607.ArticlePubMed
- 47. Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci 2008;13:2614–2632.ArticlePubMed
- 48. Pereira GS, Rossato JI, Sarkis JJ, Cammarota M, Bonan CD, Izquierdo I. Activation of adenosine receptors in the posterior cingulate cortex impairs memory retrieval in the rat. Neurobiol Learn Mem 2005;83:217–223.ArticlePubMed
- 49. Aarsland D, Laake K, Larsen JP, Janvin C. Donepezil for cognitive impairment in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry 2002;72:708–712.ArticlePubMedPMC
- 50. Kadowaki Horita T, Kobayashi M, Mori A, Jenner P, Kanda T. Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology (Berl) 2013;230:345–352.ArticlePubMed
- 51. Prediger RD, Fernandes D, Takahashi RN. Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav Brain Res 2005;159:197–205.ArticlePubMed
- 52. Prediger RD, Da Cunha C, Takahashi RN. Antagonistic interaction between adenosine A2A and dopamine D2 receptors modulates the social recognition memory in reserpine-treated rats. Behav Pharmacol 2005;16:209–218.ArticlePubMed
- 53. Giménez-Llort L, Schiffmann SN, Shmidt T, Canela L, Camón L, Wassholm M, et al. Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol Learn Mem 2007;87:42–56.ArticlePubMed
- 54. Wei CJ, Augusto E, Gomes CA, Singer P, Wang Y, Boison D, et al. Regulation of fear responses by striatal and extrastriatal adenosine A(2A) receptors in forebrain. Biol Psychiatry 2014;75:855–863.ArticlePubMed
- 55. Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, et al. Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem 2011;18:459–474.ArticlePubMedPMC
- 56. Yu C, Gupta J, Chen JF, Yin HH. Genetic deletion of A(2A) adenosine receptors in the striatum selectively impairs habit formation. J Neurosci 2009;29:15100–15103.ArticlePubMedPMC
- 57. Hewitt DJ, Ha X, Ho TW, Wolski K, Huyck S. Preladenant in patients with Parkinson’s disease and motor fluctuations: post hoc responder analysis of a phase 2, double-blind, randomized trial. Mov Disord 2012;27:S121–S121.
- 58. Stocchi F, Rascol O, Hauser RA, Huyck S, Tzontcheva A, Capece R, et al. Randomized trial of preladenant, given as monotherapy, in patients with early Parkinson disease. Neurology 2017;88:2198–2206.ArticlePubMed
- 59. Pinna A. Adenosine A(2A) receptor antagonists in Parkinson’s disease: progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued. CNS Drugs 2014;28:455–474.ArticlePubMed
- 60. Vallano A, Fernandez-Duenas V, Pedros C, Arnau JM, Ciruela F. An update on adenosine A2A receptors as drug target in Parkinson’s disease. CNS Neurol Disord Drug Targets 2011;10:659–669.ArticlePubMed
- 61. Neustadt BR, Hao J, Lindo N, Greenlee WJ, Stamford AW, Tulshian D, et al. Potent, selective, and orally active adenosine A2A receptor antagonists: arylpiperazine derivatives of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c] pyrimidines. Bioorg Med Chem Lett 2007;17:1376–1380.ArticlePubMed
- 62. Dungo R, Deeks ED. Istradefylline: first global approval. Drugs 2013;73:875–882.ArticlePubMed
- 63. Núñez F, Taura J, Camacho J, López-Cano M, Fernández-Dueñas V, Castro N, et al. PBF509, an adenosine A2A receptor antagonist with efficacy in rodent models of movement disorders. Front Pharmacol 2018;9:1200.ArticlePubMedPMC
- 64. Ren X, Chen JF. Caffeine and Parkinson’s disease: multiple benefits and emerging mechanisms. Front Neurosci 2020;14:602697.ArticlePubMedPMC
- 65. Morató X, Luján R, López-Cano M, Gandía J, Stagljar I, Watanabe M, et al. The Parkinson’s disease-associated GPR37 receptor interacts with striatal adenosine A2A receptor controlling its cell surface expression and function in vivo. Sci Rep 2017;7:9452.ArticlePubMedPMC
- 66. Luan Y, Ren X, Zheng W, Zeng Z, Guo Y, Hou Z, et al. Chronic caffeine treatment protects against α-synucleinopathy by reestablishing autophagy activity in the mouse striatum. Front Neurosci 2018;12:301.ArticlePubMedPMC
- 67. Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ 2010;17:1071–1082.ArticlePubMed
- 68. Hall CB. Comment: caffeine and PD-time to consider other interventions. Neurology 2017;89:1802.ArticlePubMed
- 69. Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A, et al. Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J Neurosci 2013;33:4329–4338.ArticlePubMedPMC
- 70. Hong SI, Kang S, Chen JF, Choi DS. Indirect medium spiny neurons in the dorsomedial striatum regulate ethanol-containing conditioned reward seeking. J Neurosci 2019;39:7206–7217.ArticlePubMedPMC
- 71. Ruby CL, Adams CA, Knight EJ, Nam HW, Choi DS. An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev 2010;3:163–174.ArticlePubMedPMC
- 72. Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, et al. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci 2004;7:855–861.ArticlePubMed
- 73. Asatryan L, Nam HW, Lee MR, Thakkar MM, Saeed Dar M, Davies DL, et al. Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res 2011;35:584–594.ArticlePubMedPMC
- 74. Kang S, Hong SI, Lee J, Peyton L, Baker M, Choi S, et al. Activation of astrocytes in the dorsomedial striatum facilitates transition from habitual to goal-directed reward-seeking behavior. Biol Psychiatry 2020;88:797–808.ArticlePubMedPMC
- 75. Kang S, Choi DS. Astrocyte adenosine signaling and neural mechanisms of goal-directed and habitual reward-seeking behaviors. Neuropsychopharmacology 2021;46:227–228.ArticlePubMed
- 76. Torti M, Vacca L, Stocchi F. Istradefylline for the treatment of Parkinson’s disease: is it a promising strategy? Expert Opin Pharmacother 2018;19:1821–1828.ArticlePubMed
- 77. Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev 1999;20:876–913.ArticlePubMed
- 78. Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–165.ArticlePubMed
- 79. Burcelin R. The gut-brain axis: a major glucoregulatory player. Diabetes Metab 2010;36 Suppl 3:S54–S58.ArticlePubMed
- 80. Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014;63:1224–1233.ArticlePubMed
- 81. Tian L, Jin T. The incretin hormone GLP-1 and mechanisms underlying its secretion. J Diabetes 2016;8:753–765.ArticlePubMed
- 82. Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 2009;150:1680–1687.ArticlePubMed
- 83. Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 2015;125:782–786.ArticlePubMedPMC
- 84. Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care 1992;15:270–276.ArticlePubMed
- 85. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301–307.ArticlePubMedPMC
- 86. Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem 2003;10:2471–2483.ArticlePubMedPMC
- 87. Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009;5:262–269.ArticlePubMed
- 88. Gheni G, Ogura M, Iwasaki M, Yokoi N, Minami K, Nakayama Y, et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep 2014;9:661–673.ArticlePubMedPMC
- 89. Peyot ML, Gray JP, Lamontagne J, Smith PJ, Holz GG, Madiraju SR, et al. Glucagon-like peptide-1 induced signaling and insulin secretion do not drive fuel and energy metabolism in primary rodent pancreatic beta-cells. PLoS One 2009;4:e6221. ArticlePubMedPMC
- 90. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157.ArticlePubMed
- 91. Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 2005;85:1303–1342.ArticlePubMed
- 92. Carlessi R, Chen Y, Rowlands J, Cruzat VF, Keane KN, Egan L, et al. GLP-1 receptor signalling promotes β-cell glucose metabolism via mTOR-dependent HIF-1α activation. Sci Rep 2017;7:2661.ArticlePubMedPMC
- 93. Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes 2017;10:123–139.ArticlePubMedPMC
- 94. Buse JB, Peters A, Russell-Jones D, Furber S, Donsmark M, Han J, et al. Is insulin the most effective injectable antihyperglycaemic therapy? Diabetes Obes Metab 2015;17:145–151.ArticlePubMed
- 95. Alexopoulos AS, Buse JB. Initial injectable therapy in type 2 diabetes: key considerations when choosing between glucagon-like peptide 1 receptor agonists and insulin. Metabolism 2019;98:104–111.ArticlePubMedPMC
- 96. Fortin SM, Chartoff EH, Roitman MF. The aversive agent lithium chloride suppresses phasic dopamine release through central GLP-1 receptors. Neuropsychopharmacology 2016;41:906–915.ArticlePubMed
- 97. Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A 2009;106:1285–1290.ArticlePubMedPMC
- 98. Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1664–1675.ArticlePubMedPMC
- 99. Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH. A new treatment strategy for Parkinson’s disease through the gut–brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplant 2017;26:1560–1571.ArticlePubMedPMC
- 100. Li Y, Vaughan KL, Tweedie D, Jung J, Kim HK, Choi HI, et al. Pharmacokinetics of Exenatide in nonhuman primates following its administration in the form of sustained-release PT320 and Bydureon. Sci Rep 2019;9:17208.ArticlePubMedPMC
- 101. Yu SJ, Chen S, Yang YY, Glotfelty EJ, Jung J, Kim HK, et al. PT320, sustained release Exendin-4, mitigates L-DOPA-induced dyskinesia in a rat 6-hydroxydopamine model of Parkinson’s disease. Front Neurosci 2020;14:785.ArticlePubMedPMC
- 102. Muscogiuri G, Cignarelli A, Giorgino F, Prodam F, Santi D, Tirabassi G, et al. GLP-1: benefits beyond pancreas. J Endocrinol Invest 2014;37:1143–1153.ArticlePubMed
- 103. Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, et al. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015;156:255–267.ArticlePubMed
- 104. Hölscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol 2014;221:T31–T41.ArticlePubMed
- 105. Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab 2016;24:15–30.ArticlePubMed
- 106. Mora PF, Johnson EL. Cardiovascular outcome trials of the incretin-based therapies: what do we know so far? Endocr Pract 2017;23:89–99.ArticlePubMed
- 107. Manigault KR, Thurston MM. Liraglutide: a glucagon-like peptide-1 agonist for chronic weight management. Consult Pharm 2016;31:685–697.ArticlePubMed
- 108. Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest 2013;123:2730–2736.ArticlePubMedPMC
- 109. Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 2012;13:33.ArticlePubMedPMC
- 110. Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther 2002;300:958–966.ArticlePubMed
- 111. Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 2008;86:326–338.ArticlePubMed
- 112. Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis 2014;4:337–344.ArticlePubMed
- 113. Athauda D, Gulyani S, Karnati HK, Li Y, Tweedie D, Mustapic M, et al. Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: a secondary analysis of the exenatide-PD trial. JAMA Neurol 2019;76:420–429.ArticlePubMedPMC
- 114. Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012;338:949–953.ArticlePubMedPMC
- 115. Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016;353:aah3374.ArticlePubMedPMC
- 116. Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, et al. Human alphasynuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A 2002;99:8968–8973.ArticlePubMedPMC
- 117. Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 2016;21:802–818.ArticlePubMed
- 118. Nazario LR, da Silva RS, Bonan CD. Targeting adenosine signaling in Parkinson’s disease: from pharmacological to non-pharmacological approaches. Front Neurosci 2017;11:658.ArticlePubMedPMC
- 119. Stacy M, Silver D, Mendis T, Sutton J, Mori A, Chaikin P, et al. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology 2008;70:2233–2240.ArticlePubMed
- 120. Kondo T, Mizuno Y; Japanese Istradefylline Study Group. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol 2015;38:41–46.ArticlePubMed
- 121. Mizuno Y, Kondo T; Japanese Istradefylline Study Group. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord 2013;28:1138–1141.ArticlePubMedPMC
- 122. Pourcher E, Fernandez HH, Stacy M, Mori A, Ballerini R, Chaikin P. Istradefylline for Parkinson’s disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism Relat Disord 2012;18:178–184.ArticlePubMed
- 123. Hauser RA, Olanow CW, Kieburtz KD, Neale A, Resburg C, Maya U, et al. A phase 2, placebo-controlled, randomized, double-blind trial of tozadenant (Syn-115) in patients with Parkinson’s disease with wearing-off fluctuations on levodopa. J Neurol Sci 2013;333:e119. Article
- 124. Factor SA, Wolski K, Togasaki DM, Huyck S, Cantillon M, Ho TW, et al. Long-term safety and efficacy of preladenant in subjects with fluctuating Parkinson’s disease. Mov Disord 2013;28:817–820.ArticlePubMed
- 125. Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 2008;5:19.ArticlePubMedPMC
- 126. Chen S, Yu SJ, Li Y, Lecca D, Glotfelty E, Kim HK, et al. Post-treatment with PT302, a long-acting Exendin-4 sustained release formulation, reduces dopaminergic neurodegeneration in a 6-hydroxydopamine rat model of Parkinson’s disease. Sci Rep 2018;8:10722.ArticlePubMedPMC
- 127. Liu W, Jalewa J, Sharma M, Li G, Li L, Hölscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience 2015;303:42–50.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Locomotor Behavior and Memory Dysfunction Induced by 3-Nitropropionic Acid in Adult Zebrafish: Modulation of Dopaminergic Signaling
Melissa Talita Wiprich, Rafaela da Rosa Vasques, Darlan Gusso, Gabriel Rübensam, Luiza Wilges Kist, Mauricio Reis Bogo, Carla Denise Bonan
Molecular Neurobiology.2024; 61(2): 609. CrossRef - Purinergic signaling: A gatekeeper of blood-brain barrier permeation
Yuemei Wang, Yuanbing Zhu, Junmeng Wang, Longcong Dong, Shuqing Liu, Sihui Li, Qiaofeng Wu
Frontiers in Pharmacology.2023;[Epub] CrossRef - Strategies for Drug Delivery into the Brain: A Review on Adenosine Receptors Modulation for Central Nervous System Diseases Therapy
Mercedes Fernandez, Manuela Nigro, Alessia Travagli, Silvia Pasquini, Fabrizio Vincenzi, Katia Varani, Pier Andrea Borea, Stefania Merighi, Stefania Gessi
Pharmaceutics.2023; 15(10): 2441. CrossRef - Restoring autophagic function: a case for type 2 diabetes mellitus drug repurposing in Parkinson’s disease
Marco Greco, Anas Munir, Debora Musarò, Chiara Coppola, Michele Maffia
Frontiers in Neuroscience.2023;[Epub] CrossRef - Adenosine receptors: Emerging non-opioids targets for pain medications
Soo-Min Jung, Lee Peyton, Hesham Essa, Doo-Sup Choi
Neurobiology of Pain.2022; 11: 100087. CrossRef - A2A Adenosine Receptor Antagonists: Are Triazolotriazine and Purine Scaffolds Interchangeable?
Andrea Spinaci, Catia Lambertucci, Michela Buccioni, Diego Dal Ben, Claudia Graiff, Maria Cristina Barbalace, Silvana Hrelia, Cristina Angeloni, Seyed Khosrow Tayebati, Massimo Ubaldi, Alessio Masi, Karl-Norbert Klotz, Rosaria Volpini, Gabriella Marucci
Molecules.2022; 27(8): 2386. CrossRef - Pathophysiological Role and Medicinal Chemistry of A2A Adenosine Receptor Antagonists in Alzheimer’s Disease
Stefania Merighi, Pier Andrea Borea, Katia Varani, Fabrizio Vincenzi, Alessia Travagli, Manuela Nigro, Silvia Pasquini, R. Rama Suresh, Sung Won Kim, Nora D. Volkow, Kenneth A. Jacobson, Stefania Gessi
Molecules.2022; 27(9): 2680. CrossRef - Modulation of adenosine signaling reverses 3-nitropropionic acid-induced bradykinesia and memory impairment in adult zebrafish
Melissa Talita Wiprich, Stefani Altenhofen, Darlan Gusso, Rafaela da Rosa Vasques, Rodrigo Zanandrea, Luiza Wilges Kist, Mauricio Reis Bogo, Carla Denise Bonan
Progress in Neuro-Psychopharmacology and Biological Psychiatry.2022; 119: 110602. CrossRef - A Focus on Astrocyte Contribution to Parkinson’s Disease Etiology
Giselle Prunell, Silvia Olivera-Bravo
Biomolecules.2022; 12(12): 1745. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite