Articles
- Page Path
- HOME > J Mov Disord > Volume 7(2); 2014 > Article
-
Case Report
Globus Pallidus Interna Deep Brain Stimulation in a Patient with Medically Intractable Meige Syndrome - Dae-Woong Bae1, Byung-chul Son2, Joong-Seok Kim1
-
Journal of Movement Disorders 2014;7(2):92-94.
DOI: https://doi.org/10.14802/jmd.14013
Published online: October 30, 2014
1Department of Neurology, College of Medicine, The Catholic University of Korea, Seoul, Korea
2Department of Neurosurgery, Seoul St. Mary’s Hospital and Catholic Neuroscience Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding author: Joong-Seok Kim, MD, PhD, Department of Neurology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 137-701, Korea / Tel: +82-2-2258-2815 / Fax: +82-2-599-9686 / E-mail: neuronet@catholic.ac.kr
• Received: August 1, 2014 • Revised: August 4, 2014 • Accepted: August 4, 2014
Copyright © 2014 The Korean Movement Disorder Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Medical therapies in patients with Meige syndrome, including botulinum toxin injection, have been limited because of incomplete response or adverse side effects. We evaluated a patient with Meige syndrome who was successfully treated with deep brain stimulation (DBS) in the globus pallidus interna (GPi). This case report and other previous reports suggest that bilateral GPi DBS may be an effective treatment for medically refractory Meige syndrome, without significant adverse effects.
- This case report was approved by the ethics committee of Seoul St. Mary’s Hospital, and the patient gave consent for video recording and academic use.
- The patient, a 47-year-old woman, had suffered from insidious onset of blepharospasm, oromandibular dystonia, and spasmodic dysphonia for 2 years before visiting our neurology outpatient clinic. The patient complained of decreased social activity during the course of her disease due to hyperkinesia of facial muscles and functional blindness secondary to blepharospasm. The patient was never exposed to neuroleptics, nor did she have a family history of dystonia or any perioral trauma.
- Upon examination, the patient demonstrated severe blepharospasm and oromandibular dystonia, which became aggravated when the patient attempted to open her eyes or tried to speak (Video 1 in the online-only Data Supplement). The results of neuropsychological testing for memory and frontal lobe functions were within normal limits. Evaluations were normal, including magnetic resonance imaging of the brain, serum chemistries, a complete blood count, serum ceruloplasmin, serum and urine copper levels, thyroid function tests, and genetic testing for the DYT1 mutation in the torsion A gene. The dental examination was also normal.
- The patient had been treated with 50 mg quetiapine, 8 mg trihexiphenidyl, and 40 mg baclofen, but these drugs were not effective, and side effects of somnolence were observed. Considering both the treatment-resistant symptoms and the severe disabilities, we decided to perform bilateral GPi DBS to control her dystonic symptoms.
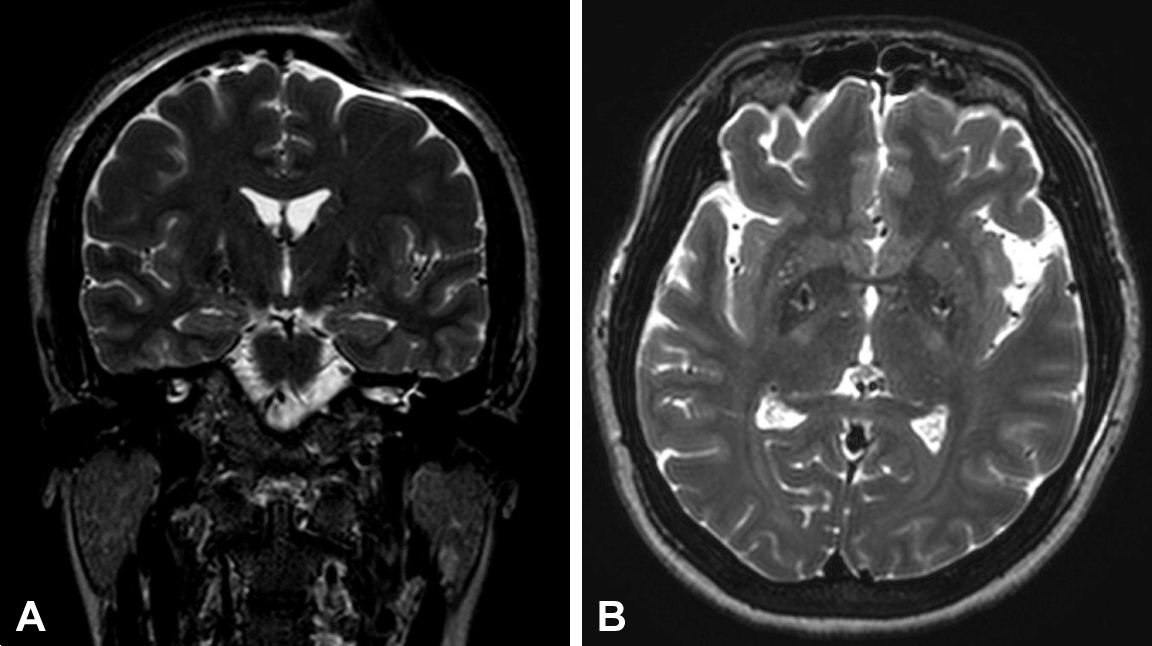
- The initial stereotactic coordinates determined by MRI-stereotactic planning were established to target the posteroventral lateral GPi. Intraoperatively, neuronal recordings were obtained using multichannel microelectrode recordings. Postoperative CT and MRI were used to verify electrode position within the GPi and to exclude asymptomatic cerebral hemorrhage (Figure 1). Postoperative programming of the DBS was performed 1 day after the staged implantation of the pulse generator. No delayed complications of the hardware occurred.
- At 2 months after the surgery, all major clinical symptoms indicated significant improvement in blepharospasm and in oromandibular and orofacial dystonia (Video 2 in the online-only Data Supplement). Speech difficulties caused by spasmodic dysphonia and/or oromandibular dystonia also responded well to pallidal stimulation. No clinically relevant bradykinesia or other abnormal movement was observed in our patient, nor were depressive mood or emotional instability. The patient was satisfied with the symptom improvement. The parameters of GPi DBS were as follows: a pulse width of 60 μsec, frequency of 130 Hz, and stimulation amplitude of 2.1 V on the right side, and a width of 90 μsec, frequency of 130 Hz, and amplitude of 1.9 V on the left side.
CASE REPORT
- The exact mechanism of the observed effect of pallidal stimulation on dystonia remains unclear, but it is possible that DBS improves dystonic symptoms by changing the plasticity in the cortical-basal ganglia circuit [10,11]. Thus, symptoms do not return immediately after the discontinuation of therapy, and continuous stimulation may not be needed in some of these patients [11].
- The most effective target area within the GPi for controlling this symptom by DBS remains under debate. Moreover, which phenotypes among the symptoms constituting Meige syndrome will respond best to DBS also remain unknown. All of these uncertainties make the publication of all previous case series and our case report important for understanding the efficacy of GPi-DBS in Meige syndrome.
- Blomstedt et al. [9] hypothesized that in patients with Meige syndrome and segmental dystonia, the GPi-DBS response may be better than in patients with purely cranio-facial dystonia. Houser and Waltz [3], in contrast, hypothesized that improvement could be predicted simply by the presence of isolated cranio-facial dystonia. Recently, the important observation was made that although disease duration can be a good predictor of the outcome of pallidal stimulation in patients with primary dystonias, no particular predictive value should be assigned to age at onset, age at surgery, severity of disease, DYT1 status or the presence of phasic or tonic involuntary movements [12].
- Numerous GPi-DBS studies have reported various adverse effects on the limbic system. Two recent reports indicated bradykinesia as a therapy-related adverse effect of pallidal neurostimulation in Meige syndrome at high voltage [13,14]. Cases of mania, depression, suicide and suicide ideations were observed in connection to pallidal stimulation. [15] Foncke et al. [16], who reported two suicides in 16 GPi-DBS treated dystonic patients, stated that these findings illustrate that there is a prominent risk of suicide after DBS of the GPi in medication-refractory dystonia.
- In summary, this case report, in combination with other previous reports, suggests that bilateral GPi DBS may be an effective treatment for medically refractory Meige syndrome, without significant adverse effects.
DISCUSSION
SUPPLEMENTARY MATERIALS
Figure 1.Postoperative coronal (A) and axial (B) T2-weighted MR images demonstrating electrode placement in a patient who underwent bilateral globus pallidus interna deep brain stimulation.


- 1. Tolosa E, Martí MJ. Blepharospasm-oromandibular dystonia syndrome (Meige’s syndrome): clinical aspects. Adv Neurol 1988;49:73–84.PubMed
- 2. Kraft SP, Lang AE. Cranial dystonia, blepharospasm and hemifacial spasm: clinical features and treatment, including the use of botulinum toxin. CMAJ 1988;139:837–844.PubMedPMC
- 3. Houser M, Waltz T. Meige syndrome and pallidal deep brain stimulation. Mov Disord 2005;20:1203–1205.ArticlePubMed
- 4. Jankovic J. Clinical features, differential diagnosis and pathogenesis of blepharospam and cranial-cervical dystonia. In: Bosniak L, editor. Blepharospasm advances in ophthalmic plastic reconstructive surgery. New York: Pergamon Press; 1998:67–82.
- 5. Isaias IU, Alterman RL, Tagliati M. Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 2009;66:465–470.ArticlePubMed
- 6. Jankovic J. Treatment of cervical dystonia with botulinum toxin. Mov Disord 2004;19 Suppl 8:S109–S115.Article
- 7. Jost WH, Kohl A. Botulinum toxin: evidence-based medicine criteria in blepharospasm and hemifacial spasm. J Neurol 2001;248 Suppl 1:21–24.ArticlePubMed
- 8. Tronnier VM, Fogel W. Pallidal stimulation for generalized dystonia. Report of three cases. J Neurosurg 2000;92:453–456.ArticlePubMed
- 9. Blomstedt P, Tisch S, Hariz MI. Pallidal deep brain stimulation in the treatment of Meige syndrome. Acta Neurol Scand 2008;118:198–202.ArticlePubMed
- 10. Vidailhet M, Grabli D, Roze E. Pathophysiology of dystonia. Curr Opin Neurol 2009;22:406–413.ArticlePubMed
- 11. Tisch S, Rothwell JC, Limousin P, Hariz MI, Corcos DM. The physiological effects of pallidal deep brain stimulation in dystonia. IEEE Trans Neural Syst Rehabil Eng 2007;15:166–172.ArticlePubMed
- 12. Isaias IU, Alterman RL, Tagliati M. Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration. Brain 2008;131(Pt 7):1895–1902.ArticlePubMedPDF
- 13. Ostrem JL, Marks WJ Jr, Volz MM, Heath SL, Starr PA. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome). Mov Disord 2007;22:1885–1891.ArticlePubMed
- 14. Berman BD, Starr PA, Marks WJ Jr, Ostrem JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotact Funct Neurosurg 2009;87:37–44.ArticlePubMedPMC
- 15. Saleh C, Okun MS. Clinical review of deep brain stimulation and its effects on limbic basal ganglia circuitry. Front Biosci 2008;13:5708–5731.ArticlePubMed
- 16. Foncke EM, Schuurman PR, Speelman JD. Suicide after deep brain stimulation of the internal globus pallidus for dystonia. Neurology 2006;66:142–143.ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Pallidal versus subthalamic deep brain stimulation for Meige syndrome: A systematic review and meta-analysis
Xin Wu, Tao Xue, Shiqing Pan, Weikang Xing, Chuanjun Huang, Jianguo Zhang, Guozheng Zhao
Heliyon.2024; 10(6): e27945. CrossRef - Bilateral pallidal DBS for blepharospasm: A case report and review of the literature
Joshua Lucas, Dorian Kusyk, Donald Whiting
Surgical Neurology International.2022; 13: 200. CrossRef - Blepharospasm, Oromandibular Dystonia, and Meige Syndrome: Clinical and Genetic Update
Hongying Ma, Jian Qu, Liangjun Ye, Yi Shu, Qiang Qu
Frontiers in Neurology.2021;[Epub] CrossRef - Pallidal versus subthalamic deep-brain stimulation for meige syndrome: a retrospective study
Jiayu Liu, Hu Ding, Ke Xu, Ruen Liu, Dongliang Wang, Jia Ouyang, Zhi Liu, Zeyu Miao
Scientific Reports.2021;[Epub] CrossRef - Predictive factors for long-term clinical outcomes of deep brain stimulation in the treatment of primary Meige syndrome
Xin Wang, Zhiqi Mao, Zhiqiang Cui, Xin Xu, Longsheng Pan, Shuli Liang, Zhipei Ling, Xinguang Yu
Journal of Neurosurgery.2020; 132(5): 1367. CrossRef - Long-Term Efficacy of Deep Brain Stimulation of Bilateral Globus Pallidus Internus in Primary Meige Syndrome
Hong Tian, Yanbing Yu, Xueke Zhen, Li Zhang, Yue Yuan, Bo Zhang, Liang Wang
Stereotactic and Functional Neurosurgery.2019; 97(5-6): 356. CrossRef - Meige's syndrome: History, epidemiology, clinical features, pathogenesis and treatment
Sanjay Pandey, Soumya Sharma
Journal of the Neurological Sciences.2017; 372: 162. CrossRef
Comments on this article
 KMDS
KMDS
 E-submission
E-submission
 PubReader
PubReader ePub Link
ePub Link Cite
Cite